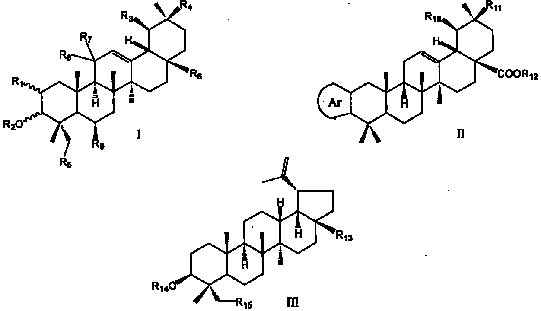
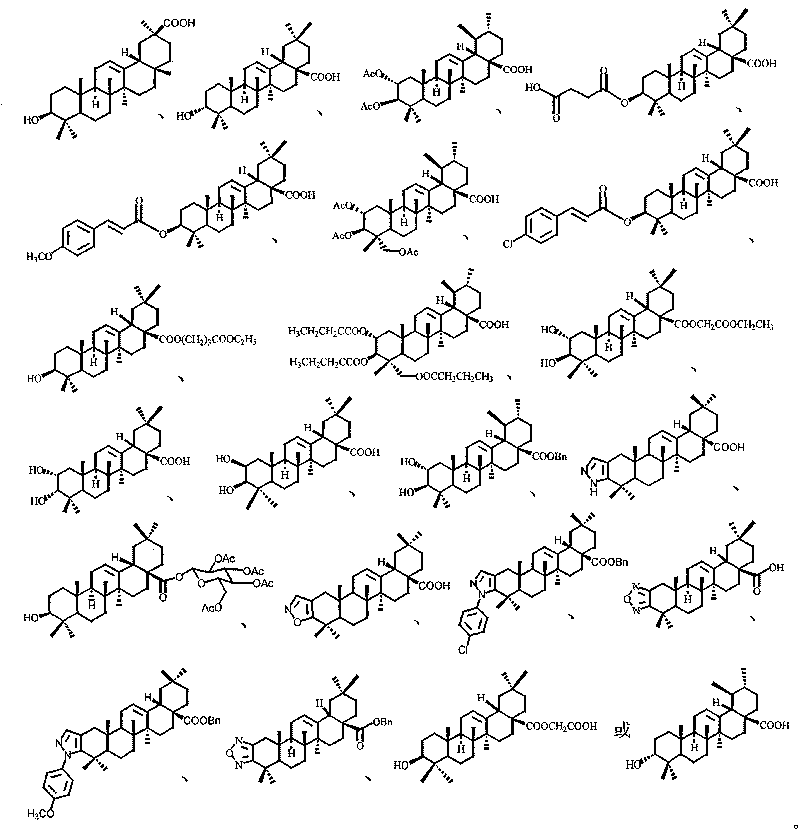
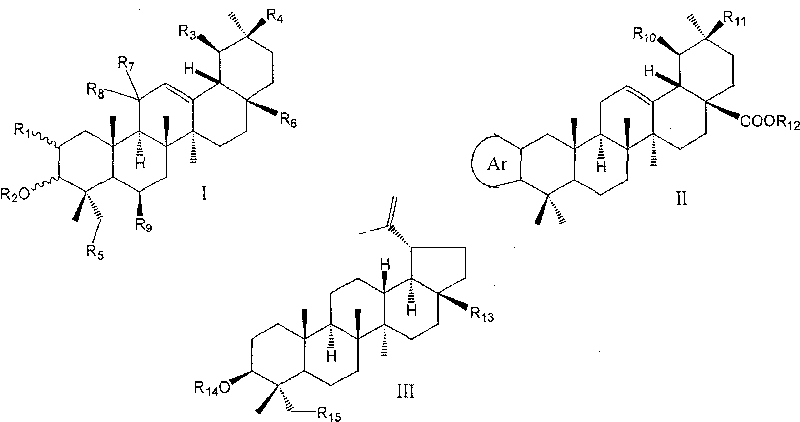
Use of pentacyclic triterpene compound as glycogen phosphorylase inhibitor
A technology of pentacyclic triterpenoids and compounds, applied in the field of medicine, can solve problems such as the limitation of oxidative energy supply
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0153] Preparation of 3-isomeric oleanolic acid
[0154] Dissolve 0.54 g of 3-carbonyloleanolic acid-28-benzyl ester (9a) in 14 mL of anhydrous isopropanol, add aluminum isopropoxide (24.7%, 2.5 g, commercial product) and a catalytic amount of AlCl 3 , the oil bath was refluxed at about 95°C. After six days, the raw materials had not completely disappeared, and the reflux was stopped. After cooling, 100 mL of 1N hydrochloric acid was slowly added dropwise to the reaction liquid, extracted with 40 mL of ethyl acetate, washed with saturated sodium bicarbonate solution, and finally Wash with saturated brine to obtain an ethyl acetate layer, anhydrous Na 2 SO 4 Drying, evaporation to dryness, and flash column chromatography (petroleum ether: ethyl acetate = 20:1) gave 0.18 g (33.2%) of 3α-hydroxyoleanolic acid-28-benzyl ester.
[0155] Add 8 mL of tetrahydrofuran to 3α-hydroxyoleanolic acid-28-benzyl ester (0.12 g), add 10% Pd / C (0.02 g), and hydrogenate at room temperature and ...
Embodiment 2
[0157] Preparation of 3-isomeric ursolic acid
[0158] With 3-carbonyl ursolic acid-28-benzyl ester (9b) as raw material, 3α-hydroxy ursolic acid was obtained with reference to the method of Example 1.
Embodiment 3
[0160] Preparation of oleanolic acid-28-methyl ester
[0161] Oleanolic acid (5.0g) was dissolved in 50mL of anhydrous DMF, adding K 2 CO 3 (3.0 g) and MeI (0.82 mL), stirred at room temperature for 2.5 hours. After dilution with water, it was extracted with ethyl acetate, and the combined ethyl acetate layer was washed successively with 1 mol / L aqueous hydrochloric acid solution and saturated aqueous sodium chloride solution, and dried over anhydrous sodium sulfate. After distilling off the solvent, 5.36 g of light yellow crude product of methyl oleanolic acid was obtained. Get 0.25g of crude product through silica gel column chromatography (eluent, petroleum ether: ethyl acetate=6:1) to obtain pure product methyl oleanate 0.23g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com