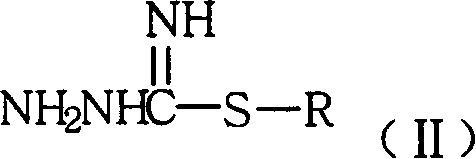Preparation method of tegaserod
A technology for tegaserod and compound, applied in the field of tegaserod, can solve the problems of complicated operation, difficult to expand production, difficult to purify, etc., and achieve the effects of simple operation method, easy industrialization, and pollution reduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
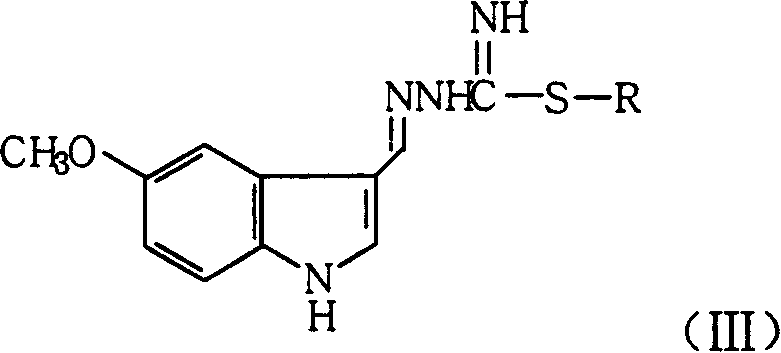
[0037] Preparation of 1-(5-methoxy-1H-indole-3-methylene)-3-methylthioguanidine hydroiodide
[0038] Mix and stir 17.5g (0.1mol) of 5-methoxyindole-3-aldehyde and 400mL of methanol, add 23.3g (0.1mol) of S-methylthiosemicarbazide hydroiodide in portions, and acidify with concentrated hydrochloric acid ( PH3~4), react at room temperature for 3 hours. After the reaction was completed, the solvent was distilled off, 200 mL of ethyl acetate was added to the residual solid, and 32.7 g of an off-white solid was obtained by filtration, yield: 83.9%. Analytical samples were recrystallized from ethanol.
[0039] Anal.:
[0040] Calcd (C 12 h 14 N 4 OS.HI): C, 36.9; H, 3.8; N, 14.4; S, 8.2.
[0041] Found: C, 36.2; H, 3.7; N, 14.1; S, 7.9.
Embodiment 2
[0043] Preparation of 1-(5-methoxy-1H-indole-3-methylene)-3-ethylthioguanidine hydrobromide
[0044] Mix and stir 11.5g (0.066mol) of 5-methoxyindole-3-aldehyde and 320mL of methanol, add 13.2g (0.066mol) of S-ethylthiosemicarbazide hydrobromide in portions, and acidify with concentrated hydrochloric acid ( PH3~4), react at room temperature for 3 hours. After the reaction was completed, the solvent was evaporated, 130 mL of ethyl acetate was added to the residual solid, and 19.9 g of off-white solid was obtained by filtration, yield: 84.9%. Analytical samples were recrystallized from ethanol.
[0045] Anal.:
[0046] Calcd (C 13 h 16 N 4 OS.HBr): C, 43.9; H, 4.7; N, 15.8; S, 9.0.
[0047] Found: C, 43.7; H, 4.5; N, 15.6; S, 8.7.
Embodiment 3
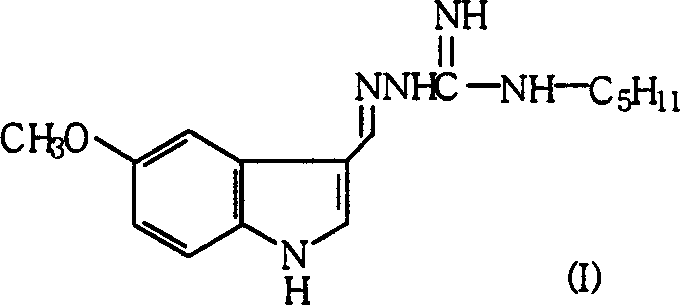
[0049] Preparation of 1-(5-methoxy-1H-indole-3-methylene)-3-pentylguanidine (tegaserod)
[0050] 31.2 g (0.08 mol) of 1-(5-methoxy-1H-indole-3-methylene)-3-methylthioguanidine hydroiodide, 7.0 g (0.08 mol) of n-pentylamine and methanol 160mL, heated to reflux, reacted for 6 hours, after the reaction was completed, distilled to dryness under reduced pressure, added 250ml of ethyl acetate to the residue, stirred, added 2mol / L sodium carbonate solution until the solids were completely dissolved, separated the organic phase, and used saturated Wash with sodium chloride solution until neutral, dry, add activated carbon for decolorization, filter, distill under reduced pressure until a solid precipitates, cool, and filter to obtain 21.6 g of white solid, yield: 89.7%. m.p.122~124℃.
[0051] Related substances: ≤0.1%.
[0052]HPLC conditions: chromatographic column: octadecylsilane bonded silica gel as filler, particle size 5 μm, specification 150×4.0 mm stainless steel column (Shi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com