Compositions and methods for augmenting kidney function
A composition and technology for renal function, applied in the field of enhancing renal function, a composition for enhancing renal function, restoring and maintaining gastrointestinal health and delaying or eliminating the development of metabolic diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
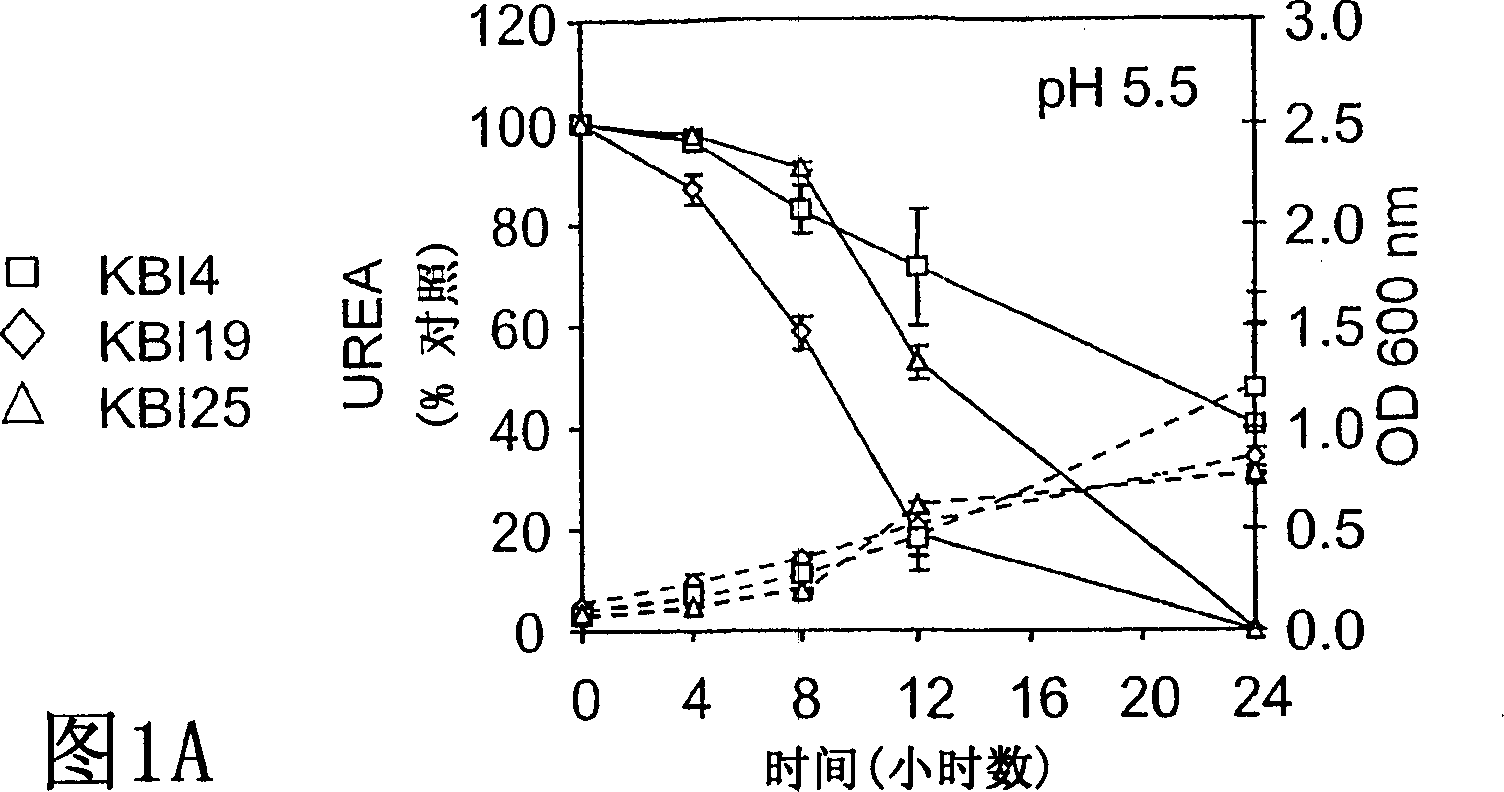
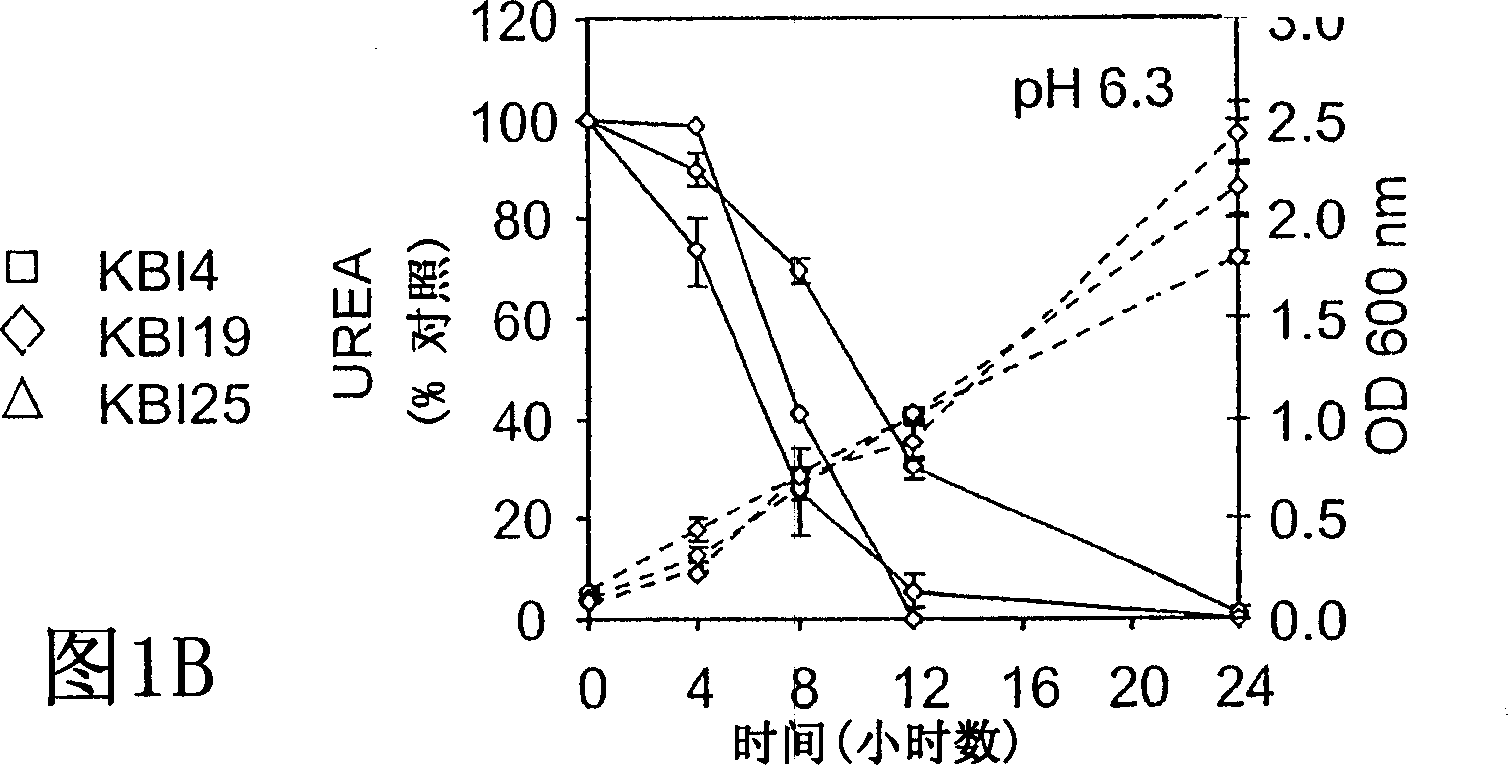
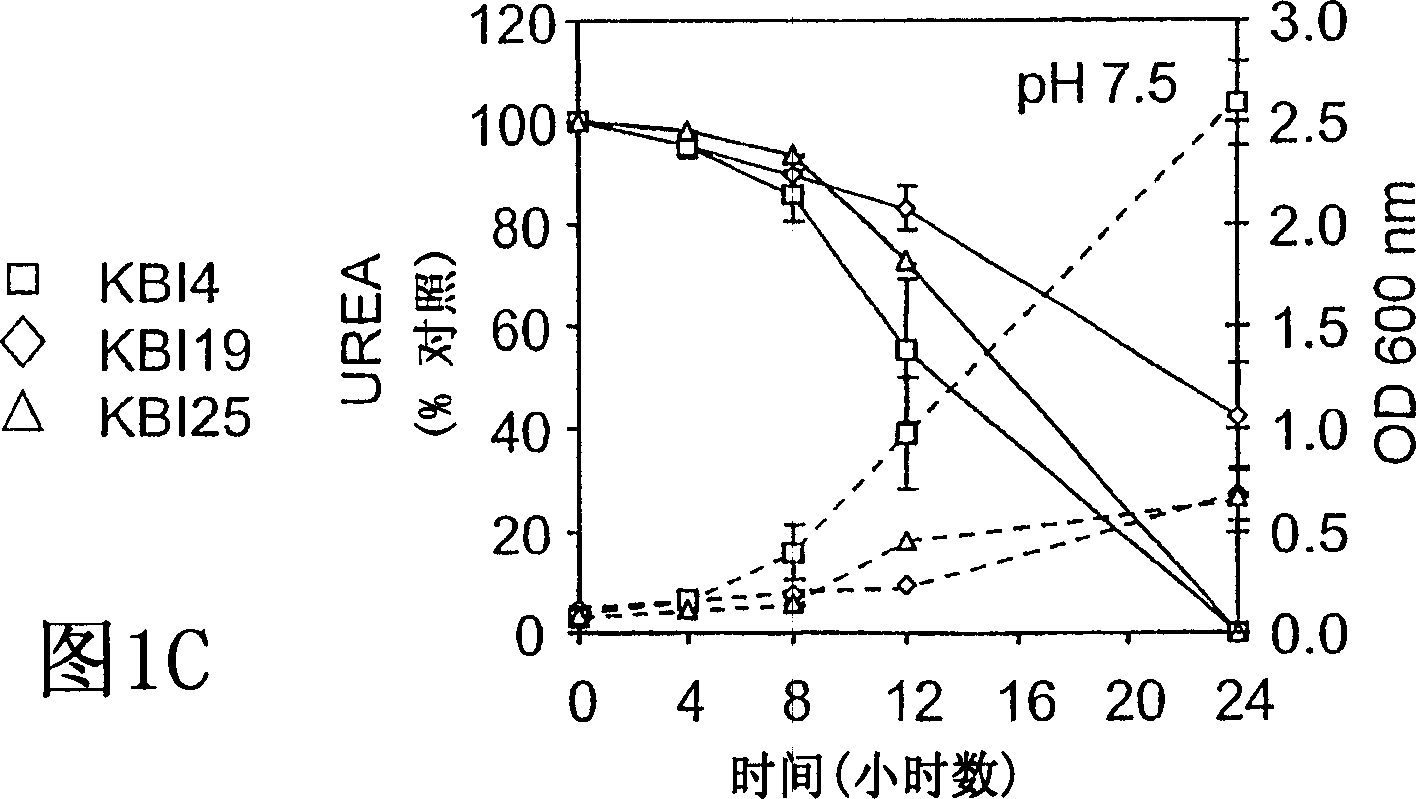
[0058] Example 1: BUN / Creatinine Reduction
[0059] Different formulations of the probiotic composition were tested with 5 / 6 nephrectomized minipigs. A total of 20 pigs were obtained over a 6 month period, 3 died postoperatively (one of the three had to be euthanized due to extreme illness) and the other two were euthanized due to acute illness possibly due to infection.
[0060] Minipigs fed formulation 1 were then split and used again to feed formulations 2A and 2B. Likewise, 2A was then used to administer formulation 2Ac. Convert using the following method. Discontinue initial bacteriological therapy and allow a 3 to 4 week rest period before switching to a new regimen. Minipigs in another group were exclusively on the assigned food regimen (3A, 3B, 4A, 5A, respectively). All screened, selected and application-specific probiotic microbial strains are generally recognized as safe (GRAS) strains identified by the FDA, except those obtained in the Bacillus pasteurian prepa...
Embodiment 2
[0064] Example 2: Preparation of Health Bars for Preparation at Room Temperature or Lower Storage Temperature
[0065] Components:
[0066] 1 cup oat bran properly ground in a dry mill or kitchen grinder, 1 / 2 cup toasted sunflower and / or sesame seeds or peanuts
[0067] 1 / 2 cup low-fat milk or soy milk powder - can be added with maltodextrin or fructose or aspartame for the right sweetness
[0068] 1 / 2 cup raisins plus 2 tablespoons carob powder.
[0069] Mix well, then add 1 / 2 cup white or brown rice, steam, dehydrate and grind and (again with food processor) add 1 / 2 cup peanut butter (how much depends on consistency)
[0070] 1 / 2 cup honey or high fructose corn syrup, about 1mL vanilla flavor (natural).
[0071] process:
[0072] Combine all ingredients and beat (adding more oat bran or rolled oats if desired) until thoroughly combined. A filter cake mixer is well suited for this application. Microbial mixed powder mixture (approximately 10 billion cfu / g) was then adde...
Embodiment 3
[0073] Example 3: Preparation of Health Bars for Non-refrigerated Conditions
[0074] Health bars that are intended for short-term consumption and are expected to be stored at room temperature (non-refrigerated conditions) are prepared at 35°C to 40°C. Combine and mix dry ingredients well (as described in Example 2). The liquid components are then added and heated to about 50°C to 60°C. The mixture is allowed to cool with subsequent mixing. The product will become more viscous. When the temperature was between 35°C and 40°C, the microbial mixture (about 10 billion cfu / g) was added. Pour the whole product into a lined trough or spread lightly with clarified butter. The product is then cooled and placed in the freezer for 12 to 24 hours until firm but easy to cut to size. The produce is cut to the desired sheet shape and size and individually wrapped and packaged.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| water activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com