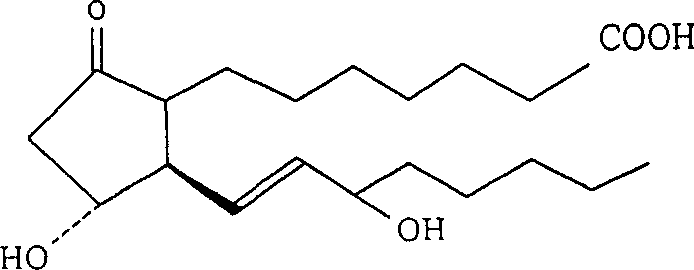
Nano emulsion injection of alprostadil and preparation method
A technology of nano-dil and alprostadil, which is applied in the direction of pharmaceutical formula, emulsion delivery, medical preparations containing active ingredients, etc., can solve the problems of poor absorption effect and large milk particles, so as to improve distribution, improve curative effect, The effect of simple and feasible quality control method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
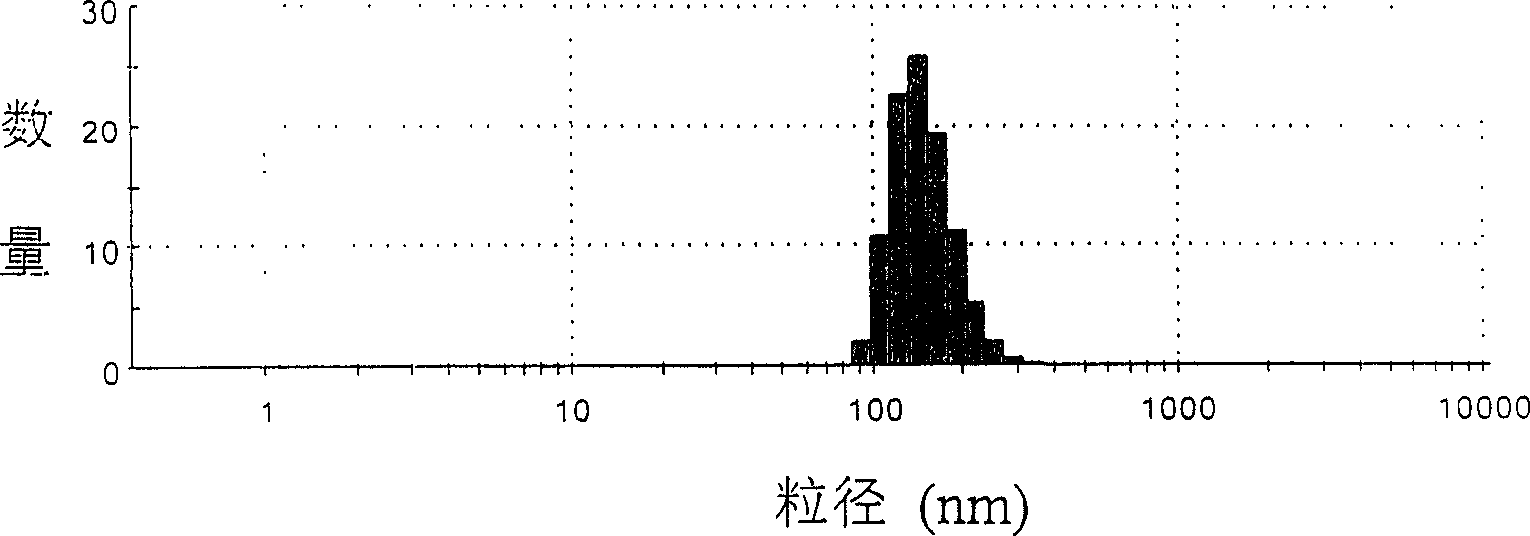
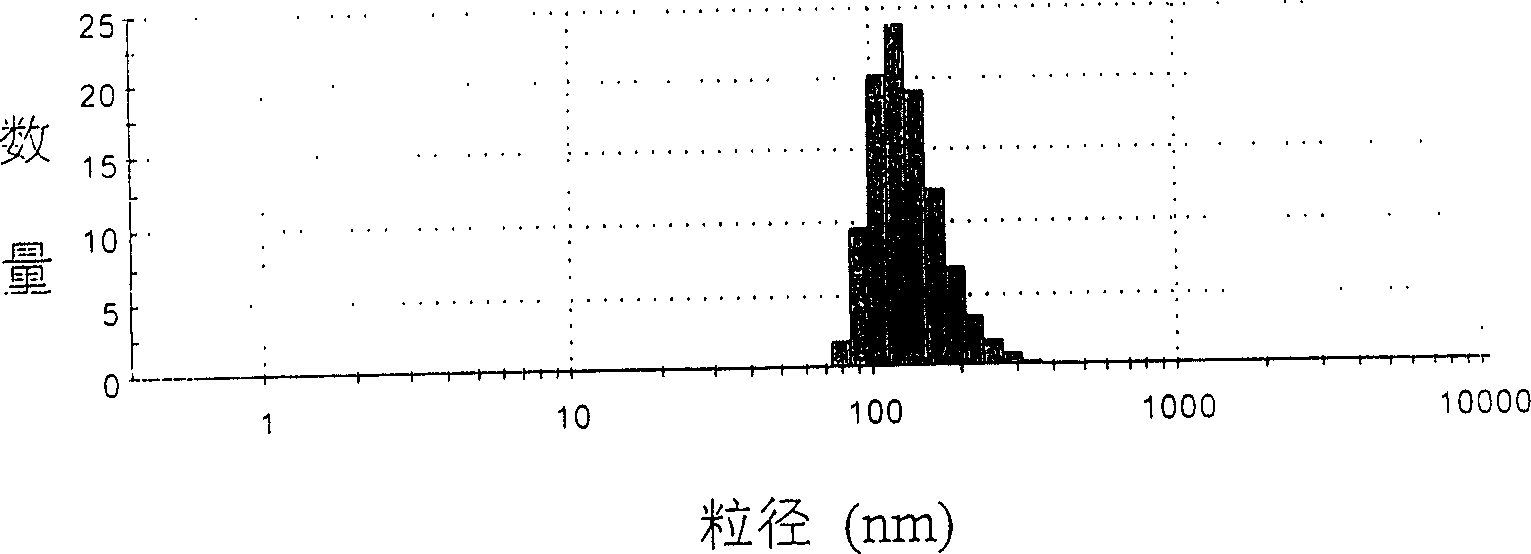
Image
Examples
Embodiment 1
[0063] Prepare raw materials, excipients and other components according to the quantity of components:
[0064] Alprostadil 0.5mg
[0065] Soybean oil for injection 3.0g
[0066] Poloxamer 188 1.0g
[0067] Lecithin for Injection 1.0g
[0068] Add water for injection to 100g
[0069] The following steps were all carried out under nitrogen protection. Add 0.5 mg of PGE1 to 3.0 g of soybean oil for injection, stir until PGE1 dissolves, and obtain solution (1), which is an oil phase. Add 1.0g of poloxamer 188 into water for injection to dissolve, under high-speed homogenization, add 1.0g of lecithin for injection, disperse for 3-6 minutes, heat the solution to 70°C, keep it warm for later use, and obtain solution (2) , is the water phase. Add the water phase solution (2) into the high-speed homogenizer, then slowly add the oil phase solution (1) into the stirring water phase, keep the temperature of the dispersion at 55°C, disperse for 3 minutes, and then add water for inje...
Embodiment 2
[0072] Prepare raw materials, excipients and other components according to the quantity of components:
[0073] Alprostadil 0.5mg
[0074] Soybean oil for injection 5.0g
[0075] Poloxamer 188 3.0g
[0076] Lecithin for Injection 1.0g
[0077] Glycerin for injection 2.25g
[0078] Oleic acid 0.5g
[0079] Add water for injection to 100g
[0080] The following steps were all carried out under nitrogen protection. Add 0.5 mg of PGE1 to 5.0 g of soybean oil for injection, stir until PGE1 dissolves, and obtain solution (1), which is an oil phase. Add 3.0g of poloxamer 188 into water for injection to dissolve, under the action of high-speed homogenization, add 2.25g of glycerin for injection, add 0.5g of oleic acid, add 1.0g of lecithin for injection, disperse for 3-6 minutes, and lower the temperature of the solution Heat to 65°C, keep warm for later use, and obtain solution (2), which is an aqueous phase. Add the water phase solution (2) into the high-speed homogenizer, t...
Embodiment 3
[0083] Prepare raw materials, excipients and other components according to the quantity of components:
[0084] Alprostadil 0.5mg
[0085] Soybean oil for injection 10.0g
[0086] Poloxamer 188 2.5g
[0087] Lecithin for Injection 2.0g
[0088] Glycerin for injection 2.5g
[0089] Oleic acid 1.0g
[0090] Add water for injection to 100g
[0091] Other steps are the same as in Example 2 to obtain the alprostadil nanoemulsion injection of the present invention. For the stability test of the alprostadil nanoemulsion injection in this example, refer to Example 7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com