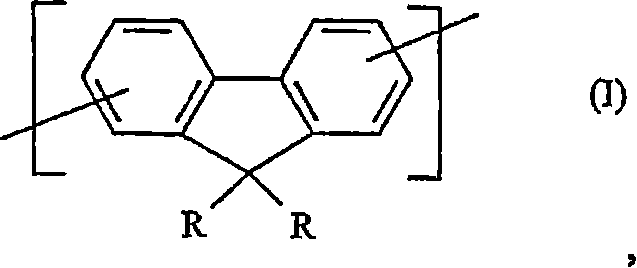
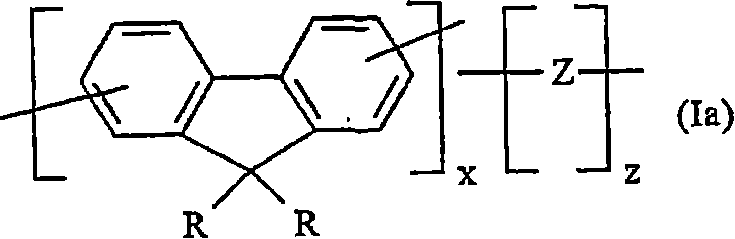
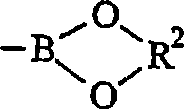
Crosslinkable substituted fluorene compounds and conjugated oligomers or polymers based thereon
A compound and substituent technology, applied in the field of electroluminescent devices, can solve the problems of limited charge transport ability and lack of conjugated polymer skeleton, and achieve the effects of reducing ionization potential, improving charge transport performance, and improving conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0128] Cross-linked polymers of the following 6 monomer units were prepared using typical Suzuki coupling reaction conditions. The various monomer unit amounts used are reported in Table 1. The resulting polymer compositions are reported in Table 2.
[0129] monomer
[0130]
[0131] monomer synthesis
[0132] 1) C6BE (2,7-bis(1,3,2-dioxaborolan-2-yl)-9,9-dioctylfluorene)
[0133] A 500 mL three-neck round bottom flask equipped with an overhead stirrer, addition funnel, and condenser connected to a nitrogen line was charged with 2,7-dibromo-9,9-dioctylfluorene (21.9 g, 40 mmol) and THF (200 mL). The mixture was stirred and cooled to about -77°C in an acetone dry ice bath. With nitrogen sparging, n-butyllithium (2.5 M in hexane, 33.6 mL, 84 mmol) was added dropwise over about 10 minutes. A yellow solution formed which gradually turned cloudy. The mixture was stirred for an additional 90 minutes at -77°C. Tris-isopropyl borate (22.6 g, 120 mmol) was then added dropwis...
Embodiment 2
[0155] The reaction scheme below discloses the preparation of triarylamine dibromide compounds containing crosslinkable benzocyclobutane functional groups, and their combination with 9,9-bis(vinylbenzyl)-2,7-fluorenyl Use in the polymerization of diboride and bis(p-bromophenyl)(p-isobutylphenyl)amine to prepare crosslinkable copolymers according to the invention.
[0156]
[0157] In this scheme, "o-tolyl" means "o-tolyl", "toluene" means "toluene", and "surfactant" means "surfactant".
[0158] A) the synthesis of diphenylbenzocyclobutaneamine
[0159] Into a 500 ml three necked round bottom flask equipped with a mechanical stirrer, nitrogen inlet and reflux condenser (with nitrogen outlet), palladium(II) acetate (196 mg, 1.20 mmol) and tris(ortho-tolyl)phosphine ( 731mg, 2.40mmol) was added to 100ml of toluene. Under nitrogen, the mixture was stirred at room temperature until the palladium catalyst dissolved and the solution turned yellow. Diphenylamine (20.0 g, 118 mmo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com