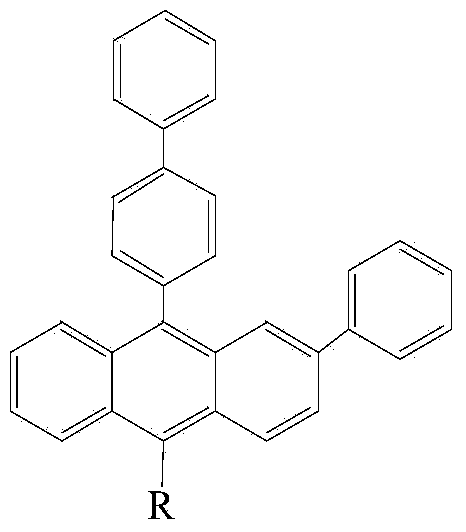
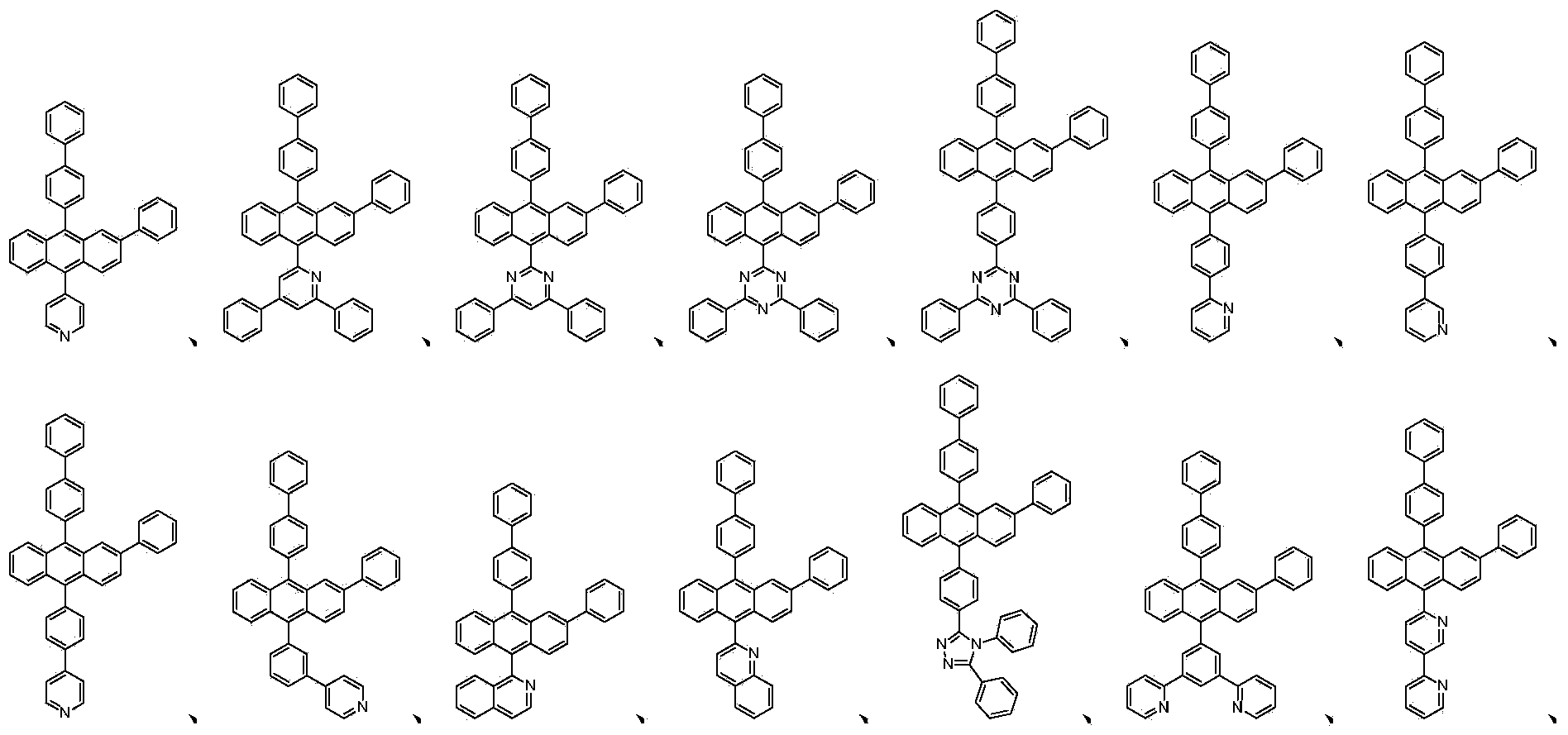
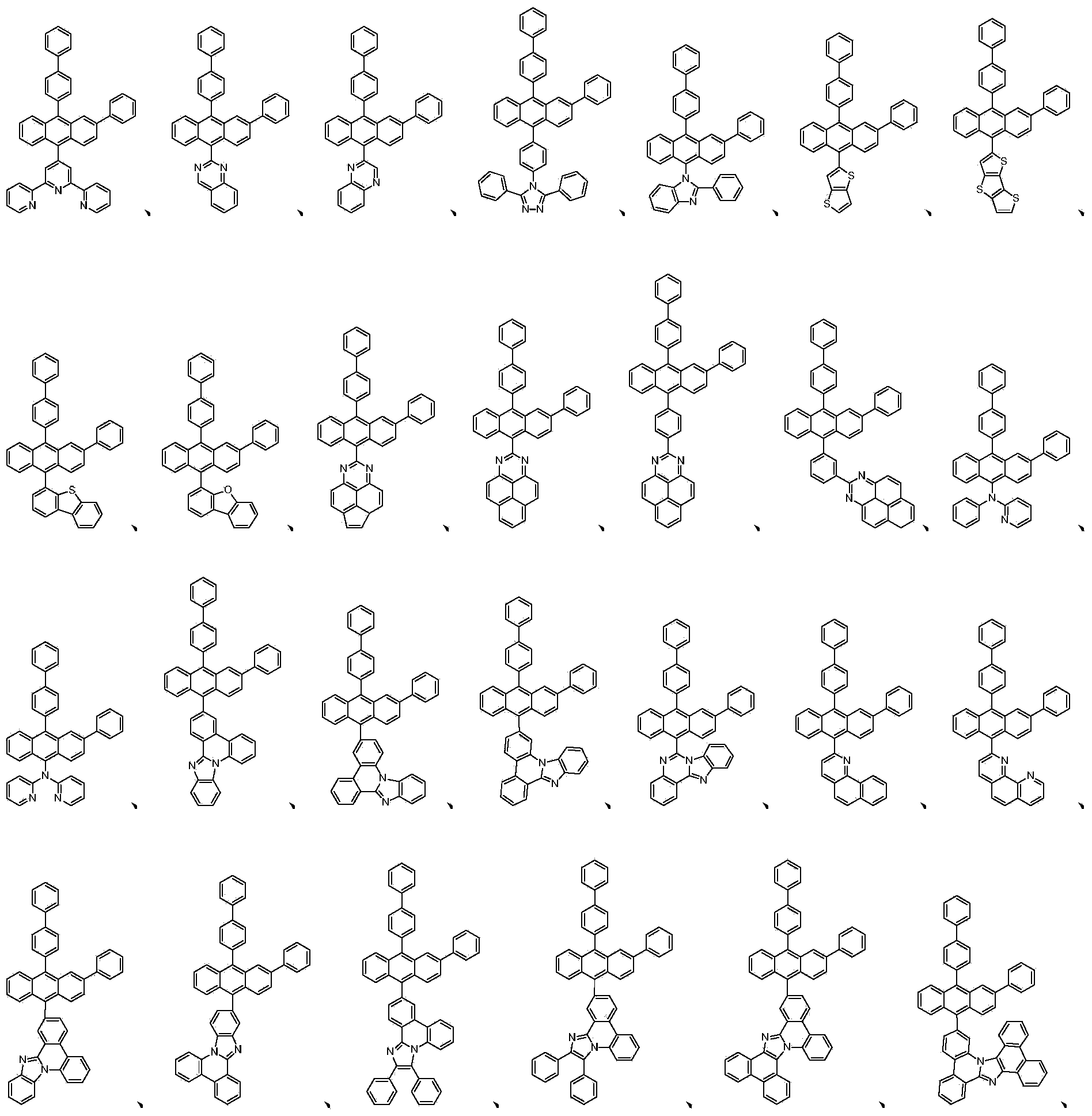Anthracene containing derivative as well as preparation method and application thereof
A derivative, anthracene-based technology, applied in the field of organic optoelectronic materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The present invention also provides a method for preparing the anthracene-containing derivatives, which comprises the following steps:
[0033] Step S1: adding an anthracene bromine-substituted compound and a boronic acid compound containing a substituent R to the reaction system;
[0034] Step S2: degassing the reaction system, and then adding a catalyst;
[0035] Step S3: Elevate the reaction temperature of the reaction system and reflux to fully react to obtain the anthracene-containing derivatives.
Embodiment 1
[0037] In order to illustrate the preparation method in the present invention in more detail, the anthracene-containing derivatives designated as 31 below As an example to describe. Its specific reaction equation is shown in formula 1.
[0038] Formula 1
[0039]
[0040] Under nitrogen protection, add compound [31-1] 50g (103.00mmol) in formula 1 to 2L reactor, compound [31-2] 41.79g (133.90mmol), K 2 CO 3 22.77g (164.80mmol), stirred with 500mL of toluene. And when the temperature in the reactor was raised to 70° C., 3.57 g (3.09 mmol) of catalyst tetrakis(triphenylphosphine) palladium was added, and 75 mL of distilled water was added and stirred for 11 hours. Add 70mL of water to end the reaction, filter under reduced pressure to obtain the crude product of the target derivative, wash three times with distilled water, then recrystallize with acetone, toluene, tetrahydrofuran solution to obtain a solid, then sublimate and refine it, and recrystallize toluene to obtai...
Embodiment 2
[0055] The fluorescent green host material in the comparative sample 1 is replaced by the derivatives represented by 1-48 in Table 1 to the original compound a , and the rest of the treatment process and conditions are the same, and an organic light-emitting device is prepared.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com