Glutamine synthetase and its dedicated expression engineered bacteria and uses
A technology of glutamine and synthase, applied in the direction of enzymes, bacteria, enzymes, etc., can solve the problems of decreased GS enzyme activity, decreased GS enzyme activity, loss and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1, the acquisition of glutamine synthetase site-directed mutation gene
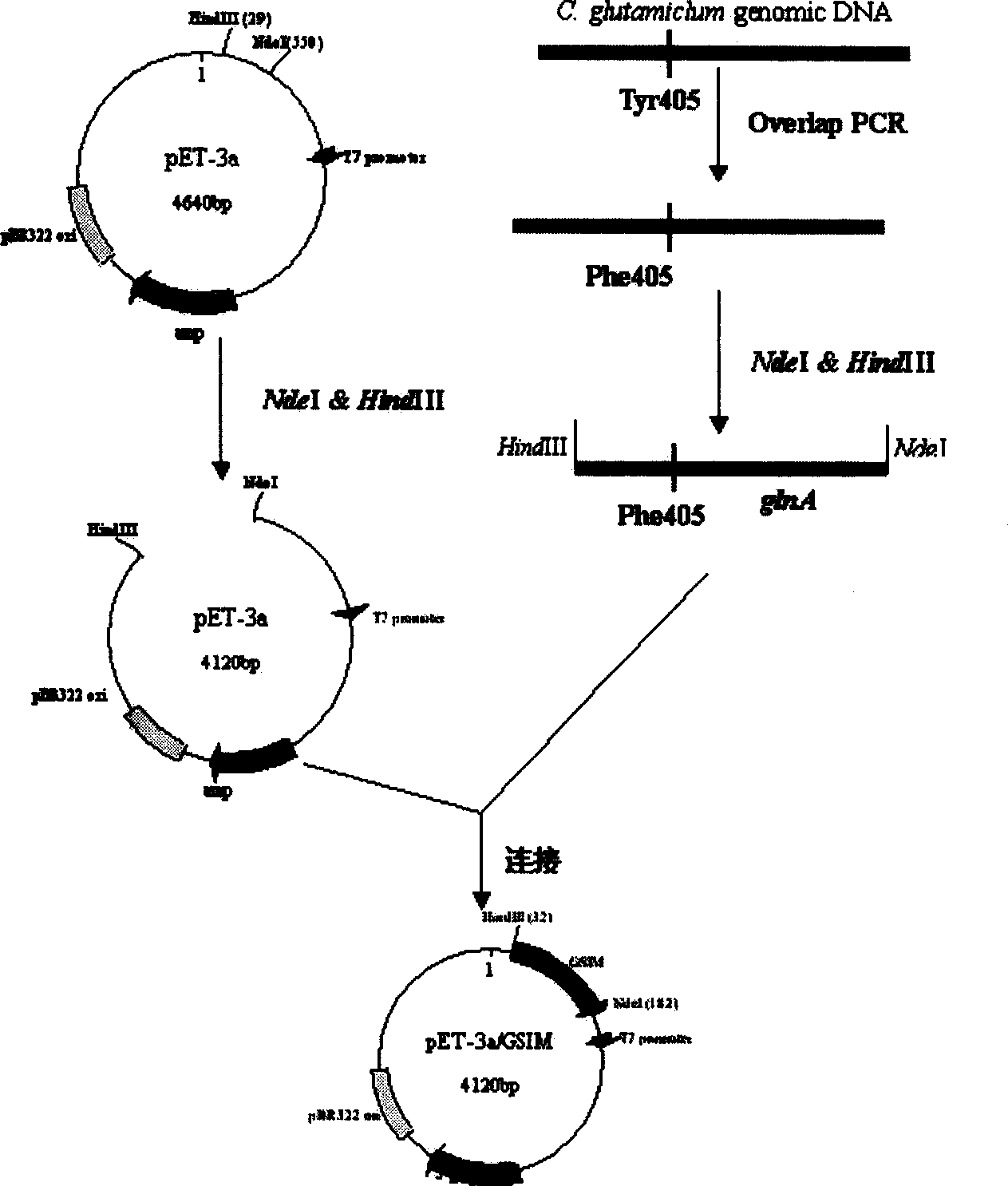
[0027] The glutamine synthetase gene (NCBIGenBank No.: Y13221) of Corynebacterium glutamicum (C. glutamicum) was subjected to site-directed mutation by overlapping PCR method, and the adenylation site tyrosine from the 1213-1215th position of the 5' end Acid codons are mutated into phenylalanine codons, and recognition sites for restriction endonucleases Nde I and Hind III are respectively introduced at both ends of the sequence. The specific method includes the following steps:
[0028] 1. The first round of amplification
[0029] Using the genomic DNA of Corynebacterium glutamicum (C. glutamicum) as a template, primer 1: 5'-ATAAGGGAGGAGTG CATATG GCGTTTGAAA-3' (the underlined base is the restriction endonuclease Nde I recognition site, SEQ ID No. 1 in the sequence listing) and primer 3: 5'-GGTAG Under the guidance of a primer pair composed of GAAGAGGTCCTTGTCCACTGGAG-3' (the base in ...
Embodiment 2
[0034] Embodiment 2, the construction of glutamine synthetase special-purpose expression engineering bacterium
[0035] 1. Construction of glutamine synthetase gene recombinant Escherichia coli expression vector
[0036] After the glutamine synthetase mutant gene amplified in Example 1 was digested with restriction endonucleases Nde I and HindIII, it was combined with the ampicillin (Amp) resistance marker-containing plasmid pET- 3a (Novagen) for ligation, the ligated product was transformed into E.coli BL21 (DE3) competent cells, and the transformant with Amp resistance was screened to obtain a recombinant expression vector containing glutamine synthetase mutant gene, which was named pET-3a / GSIM, its construction flow chart see figure 1 .
[0037] 2. Transform the recombinant expression vector pET-3a / GSIM into Escherichia coli
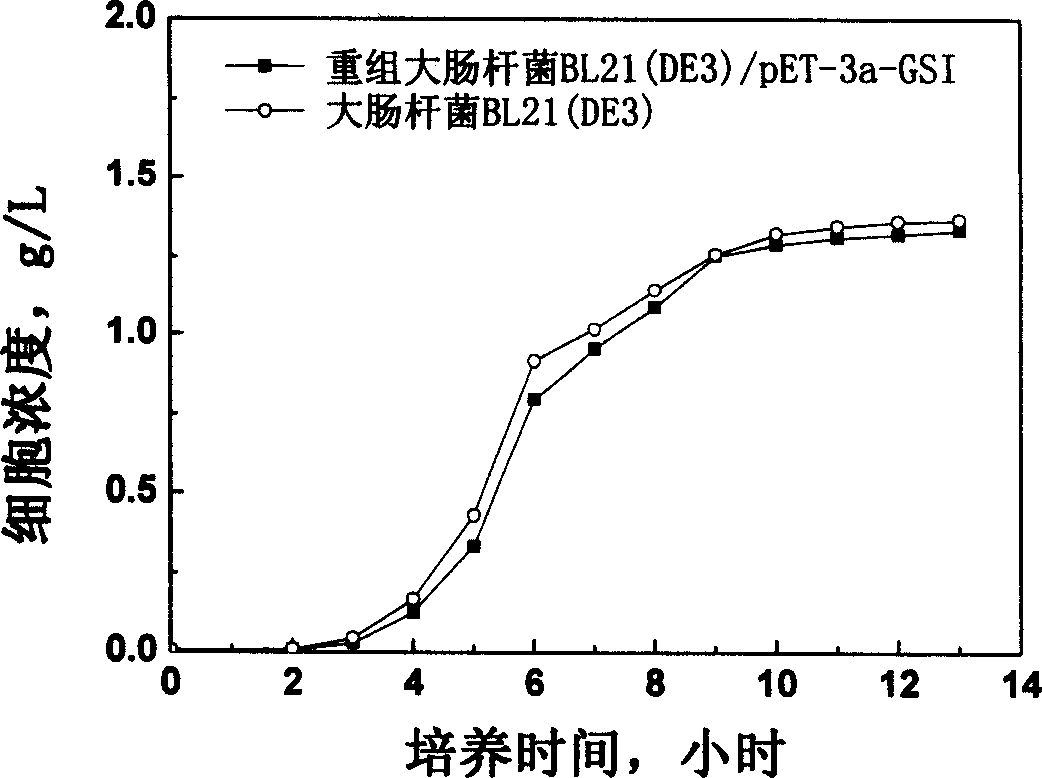
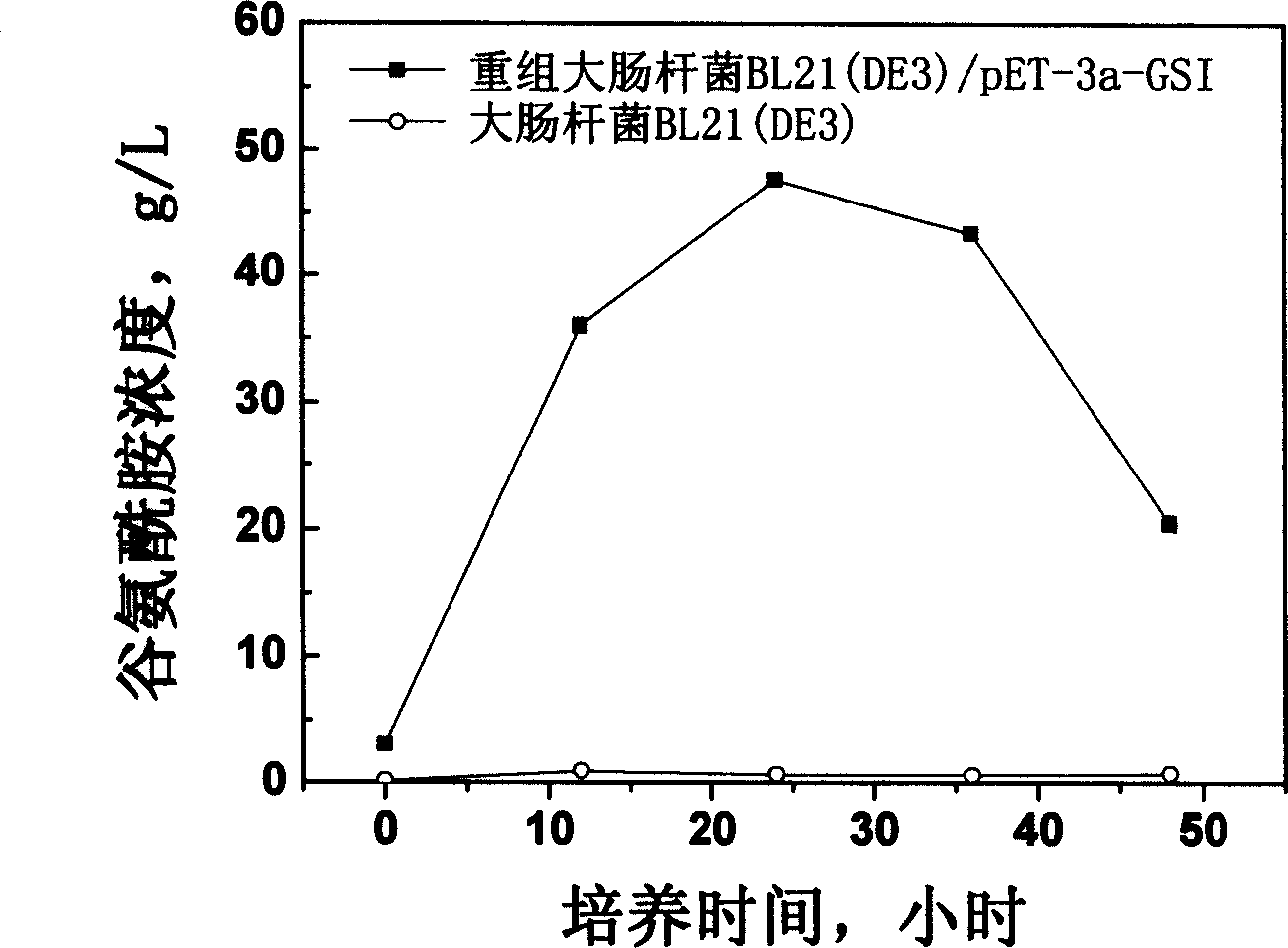
[0038] Use the recombinant expression vector pET-3a / GSIM constructed in step 1 with CaCl 2 E.coli BL21(DE3) competent cells were transformed by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com