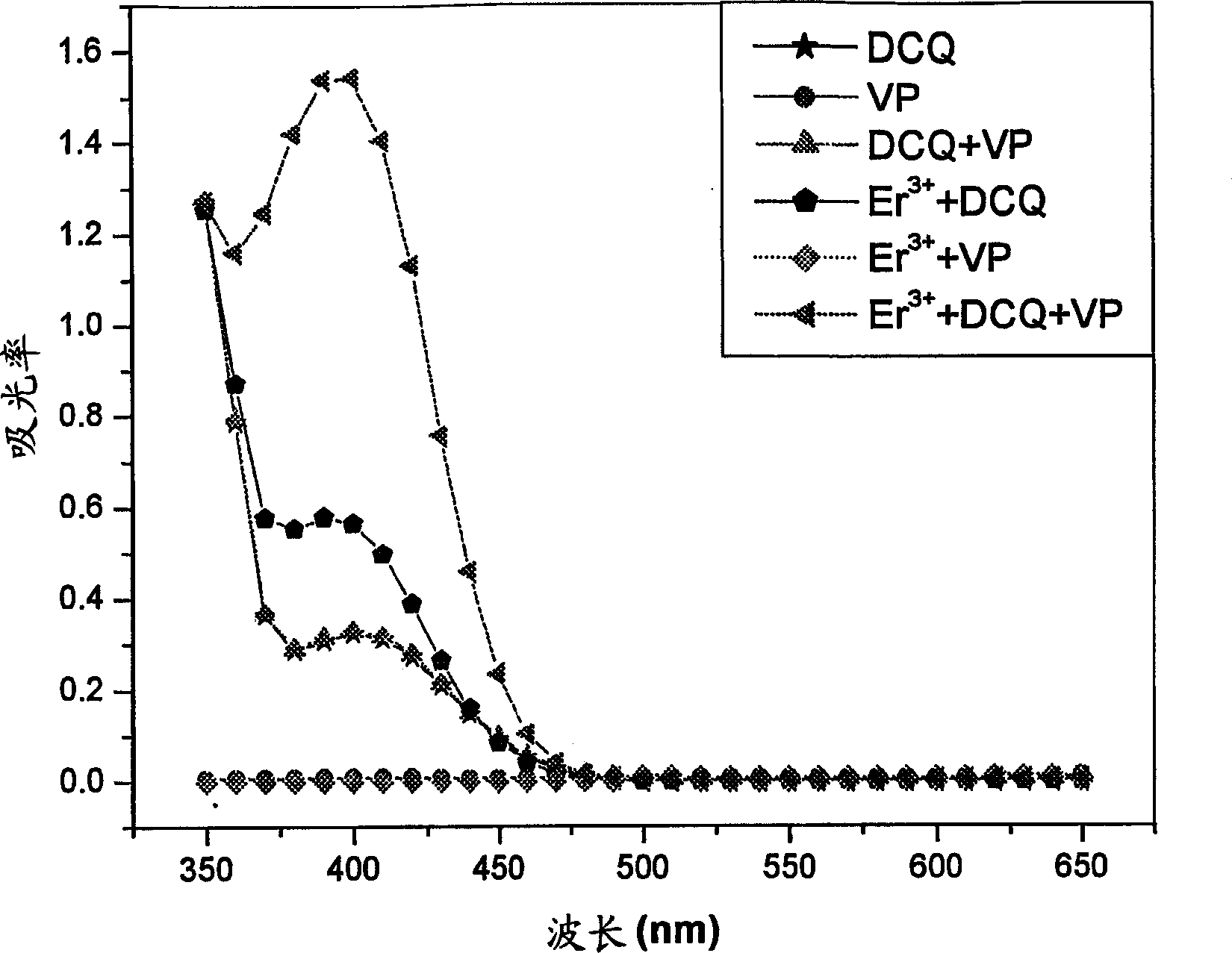
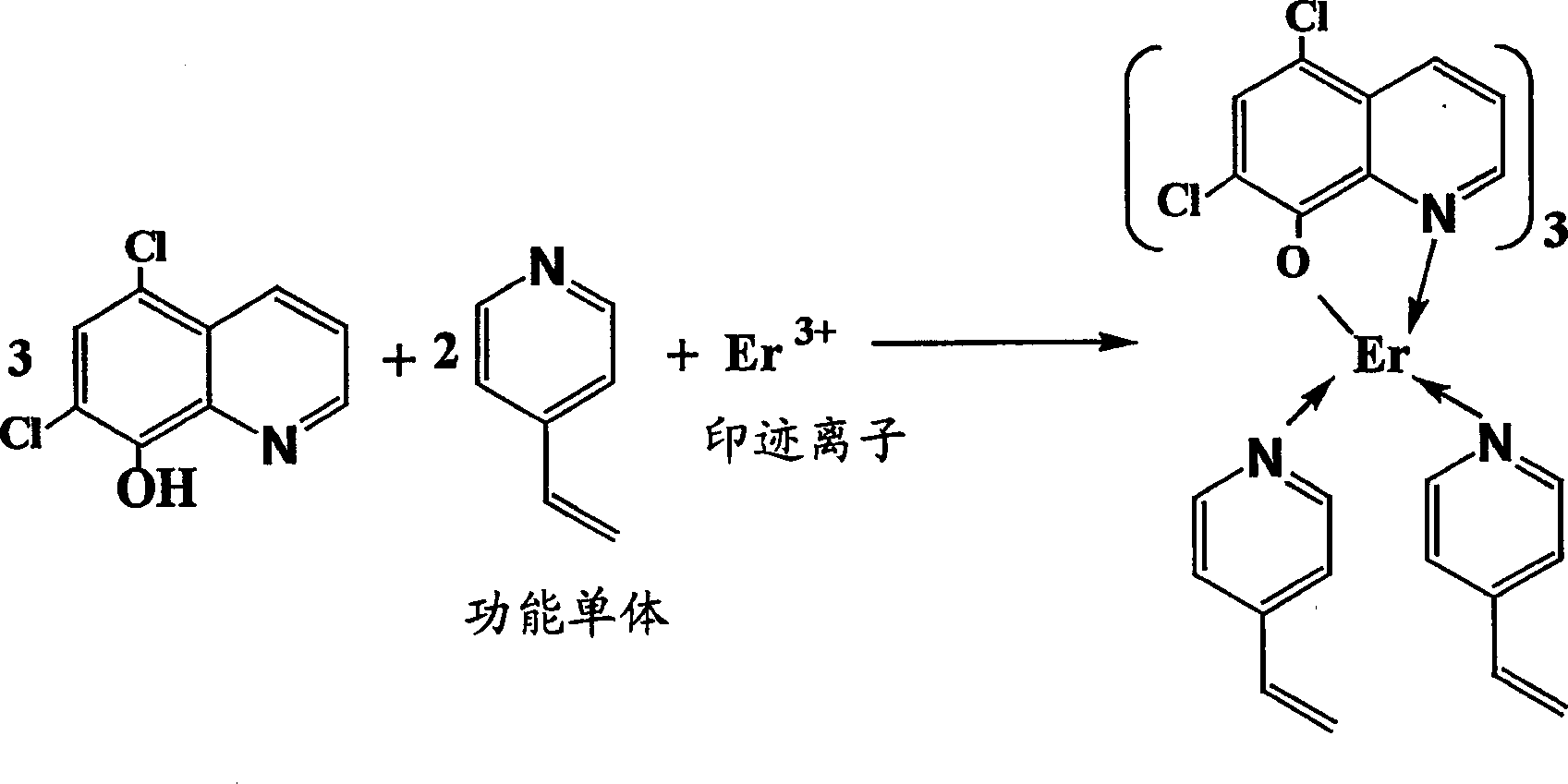
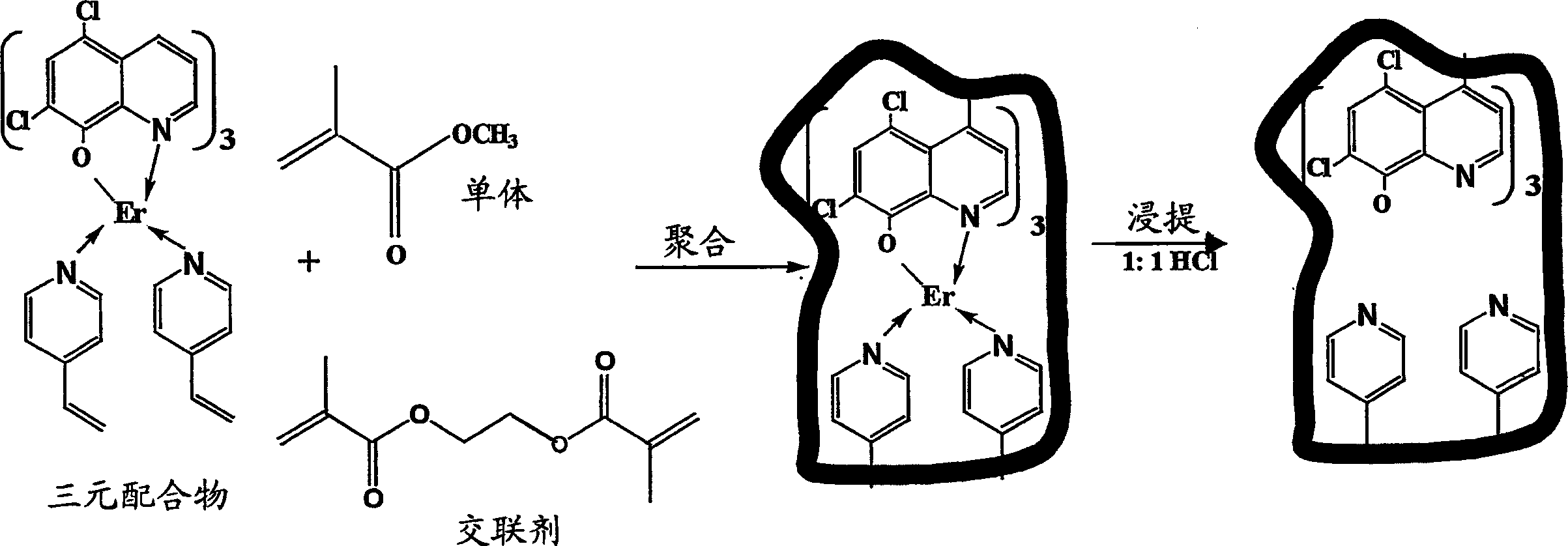
Synthesis of ion imprinted polymer particles
A technology of imprinting polymers and ion imprinting, applied in the directions of alkali metal compounds, alkali metal oxides/hydroxides, inorganic chemistry, etc., can solve the problems of uncommon separation and contact with inorganic ions, and no separation is involved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1: Gamma-radiation polymerization
[0060] In a 50ml round bottom flask, 1.0mM Erbium chloride (0.44g), 3.0mM DCQ (0.64g) and 2mM VP (0.21g) were added and dissolved in 5 or 10ml 2-methoxyethanol with stirring. 4 (0.4g) or 8 (0.8g) and 12 (1.2g) mM MMA and 16 (3.17g) or 32 (6.34g) and 48 (9.52g) mM EGDMA were added and stirred until a homogeneous solution was obtained. Transfer the monomer mixture to a test tube, cool to 0°C, and 2 Purge for 10 minutes and seal.
[0061] Application Co 60 Source, these solutions were irradiated for 4 hours under 1M rad radiation gamma-irradiation. The solid formed was washed with water and dried in an oven at 50°C. 5.70, 9.43 and 14.27 g of polymeric material were obtained with 4, 8 and 12 mM functional monomer, respectively. The polymer intercalating the erbium ions was leached with 50% (v / v) HCl while stirring for 6 hours. After drying in an oven at 50°C, 4.14, 7.52 and 11.29 g of polymeric material were obtained with 4,...
Embodiment 2
[0062] Embodiment 2: photochemical method polymerization
[0063] In a 50ml round bottom flask, 1.0mM Erbium chloride (0.44g), 3.0mM DCQ (0.64g) and 2.0mM VP (0.21g) were added and dissolved in 10ml 2-methoxyethanol with stirring. 8mM MMA (0.8g), 32mM EGDMA (6.35g) and 50mg AIBN were added and stirred until a homogeneous solution was obtained. Transfer the monomer mixture to a test tube, cool to 0°C, and 2 Purge for 10 minutes and seal. These solutions were polymerized by UV radiation (300 nm) for 4, 8 and 16 hours. The solid formed was washed with water and dried in an oven at 50°C. 7.55, 9.85 and 9.95 g of polymer material were obtained with UV radiation (300 nm) for 4, 8 and 16 h. The polymer intercalating the erbium ions was leached with 50% (v / v) HCl while stirring for 6 hours. After drying in an oven at 50° C., 5.35, 7.31 and 7.36 g of polymeric material were obtained after UV irradiation for 4, 8 and 16 h, respectively.
Embodiment 3
[0064] Example 3: thermal polymerization
[0065] In a 50ml round bottom flask, 1.0mM Erbium chloride (0.44g), 3.0mM DCQ (0.64g) and 2.0mM VP (0.21g) were added and dissolved in 10ml 2-methoxyethanol with stirring. 8.0 mM MMA (0.8 g), 8, 16 and 32 mM EGDMA (1.59, 3.17 and 6.34 g) and 50 mg AIBN were added and stirred until a homogeneous solution was obtained. The polymerization mixture was cooled to 0 °C with N 2 Purge for 10 minutes, seal and heat and stir in an oil bath at about 80°C for 2 hours. The solid formed was washed with water and dried in an oven at 50°C. 4.32, 5.50 and 8.84 g of polymer material were obtained with 50%, 66% and 80% crosslinking monomer. The polymer intercalated with erbium ions was extracted with 100 ml of 50% (v / v) HCl, stirred for 6 hours, filtered, and dried in an oven at 50°C. 2.59, 3.90 and 7.90 g of erbium ion imprinted polymer material were obtained.
[0066] Advantages of the present invention:
[0067] Liquid-liquid extraction methods...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com