Isolated phages and their use as disinfectant in food or for sanitation of factory environment
A bacteriophage, food technology, applied to bacteriophage isolates and their use as antimicrobial agents in the food field, can solve problems such as danger to infants, and achieve the effect of preventing contamination and overgrowth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: according to the screening of phage of the present invention
[0036] source
FSMCC
n°
source
FSMCC
n°
1
Factory environment
9 / 145 / 1
58
Sewage (mainly mud)
64 / 33 / 2
2
"
9 / 145 / 2
59
"
64 / 311
3
"
9 / 145 / 3
60
Sewage (channel rejection in the lake)
66 / 311 / 1
4
"
9 / 145 / 4
61
"
66 / 311 / 2
5
"
9 / 145 / 5
62
"
67 / 33 / 1
6
"
9 / 261
63
"
67 / 33 / 2
7
"
9 / 261 / 2
64
"
67 / 300 / 1
8
"
10 / 145 / 1
65
"
67 / 300 / 2
9
"
10 / 145 / 2
66
"
67 / 311 / 1
10
"
10 / 145 / 3
67
"
67 / 311 / 2
11
"
10 / 145 / 4
68
Sewage (water arrives before filtering on sand)
61 / MC9 / 1
...
Embodiment 2
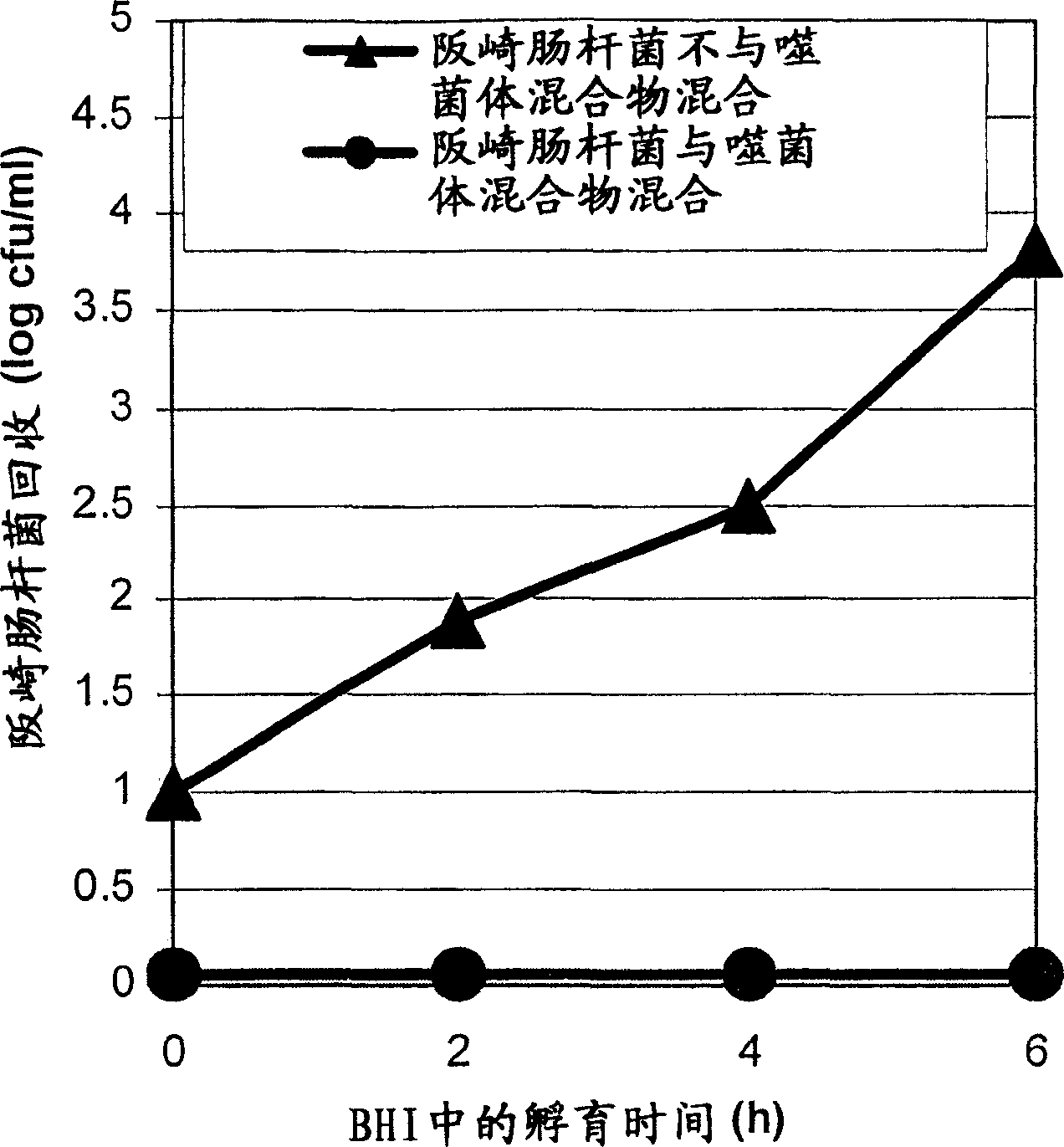
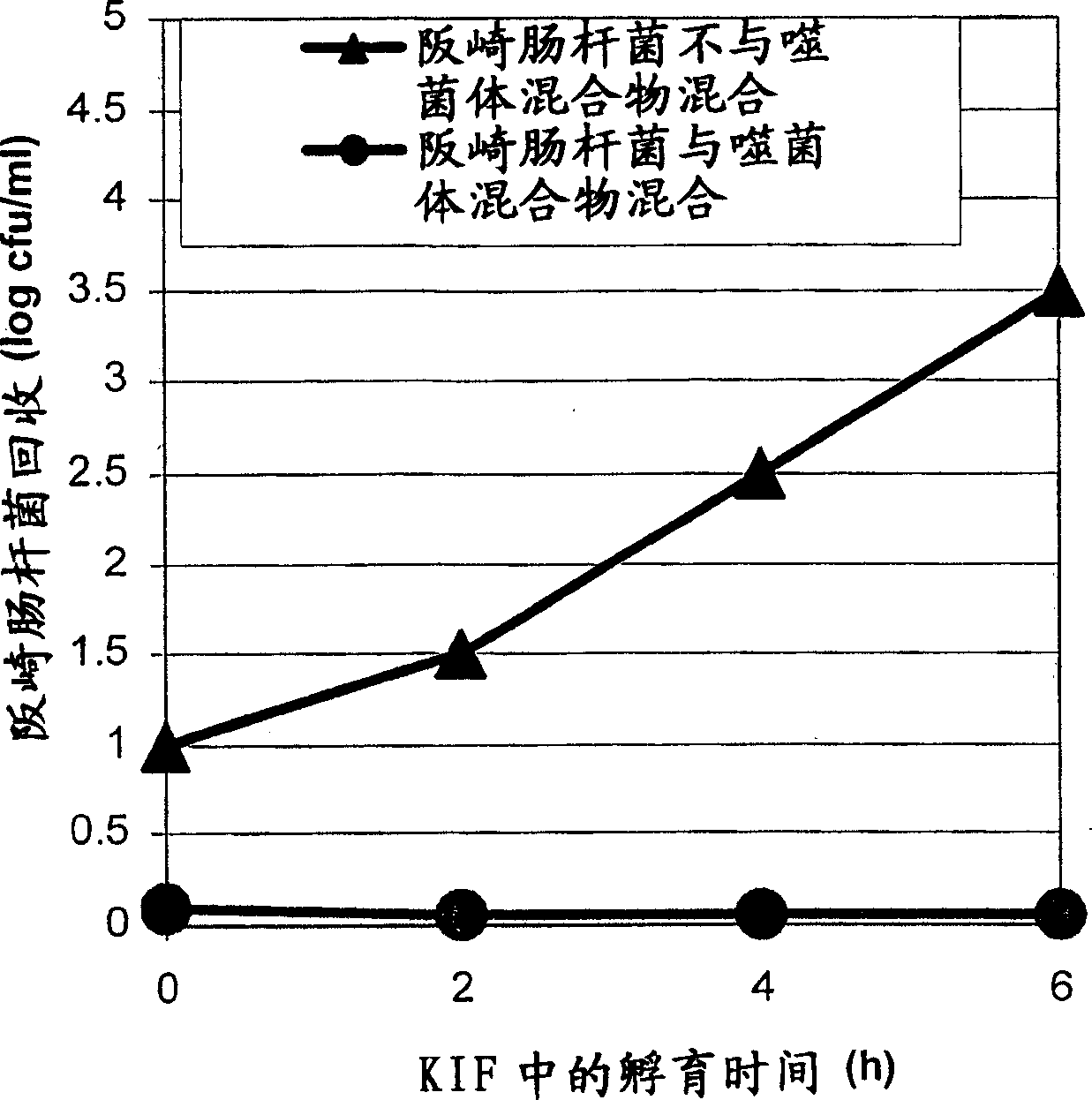
[0117] Example 2: Evaluation of the efficacy of phage mixtures in BHI and reconstituted infant formula (RIF) against a mixture of Enterobacter sakazakii strains
[0118] To assess the potency of the phage cocktail against the E. sakazakii strain mixture, the following experiments were performed.
[0119] A mixture of Enterobacter sakazakii strains FSM-145, 286, 290, 305 and 1387 / 2NL as described in Example 1 and in P. Breeuwer et al., 2003, Journal of Applied Microbiology 95: 967-973 was used. Individual overnight cultures of these strains (18 hours at 30°C) were diluted 1:1 with fresh BHI and incubated at 30°C for 2 hours. Mixing of these strains was performed by mixing equal amounts of each stock solution. Then, 100 μl of E. sakazakii mixture (prepared as described above) was used to inoculate BHI broth or RIF to a final concentration of approximately 10 cfu / ml.
[0120] FSM_phage 67 / 33 / 1 (CNCM I-3130), FSM_phage F / 316 (CNCM I-3131), FSM_phage 9 / 261 (CNCM I-3132) and FSM_p...
Embodiment 3
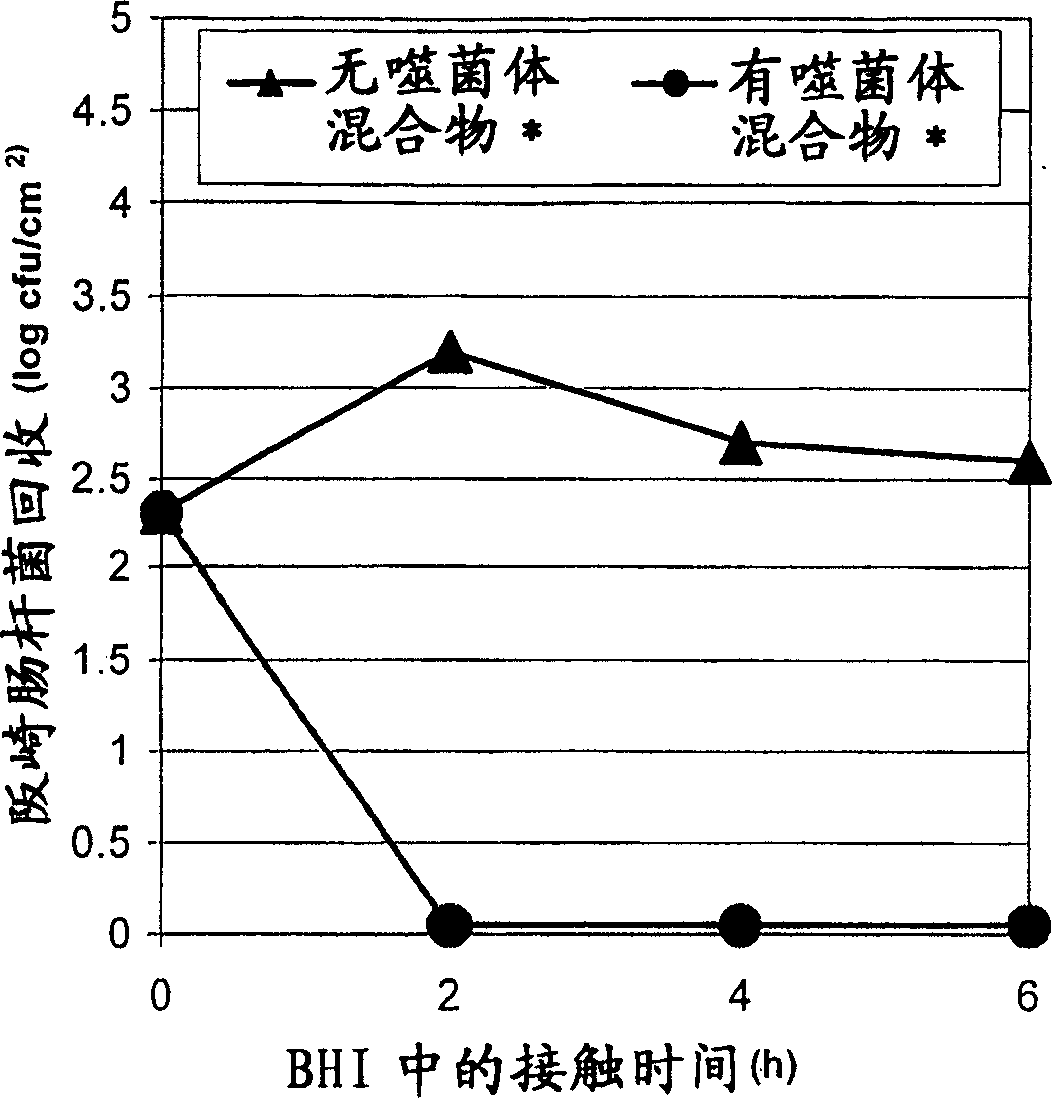
[0125] Embodiment 3: the effect of bacteriophage preparation on environmental sanitation
[0126] To assess the effect of the phage cocktail on surfaces artificially contaminated with a mixture of E. sakazakii strains, the following experiments were performed.
[0127] Step 1: Preparation of Bacterial Culture
[0128] A mixture of Enterobacter sakazakii strains (FSM-145, 286, 290, 305 and 1387 / 2NL (see Examples 1 and 2)) was used. Individual overnight cultures of these strains (18 hours at 30°C) were centrifuged at 3000 rpm for 10 minutes at 4°C. Cell pellets were harvested and diluted in BHI to achieve a cell concentration of approximately 8 logcfu / ml. Mixing of these strains was performed by mixing equal amounts of each stock solution.
[0129] Step 2: Adhesion
[0130] 1cm 2 Put the stainless steel plate into the bacterial solution prepared as in step 1. Gently shake for 1 hour at room temperature.
[0131] Step 3: Wash and Dry
[0132] To remove non-attached cells,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com