Paramagnetic metal coordination compound magnetic resonance imaging contrast medium with narrow-leaved oleaster polyose modification
A paramagnetic metal, magnetic resonance imaging technology, applied in MRI/magnetic resonance imaging contrast agents, preparations for in vivo experiments, pharmaceutical formulations, etc. The effect is not ideal and other problems, to achieve the effect of good water solubility, good selectivity, and improved imaging contrast
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
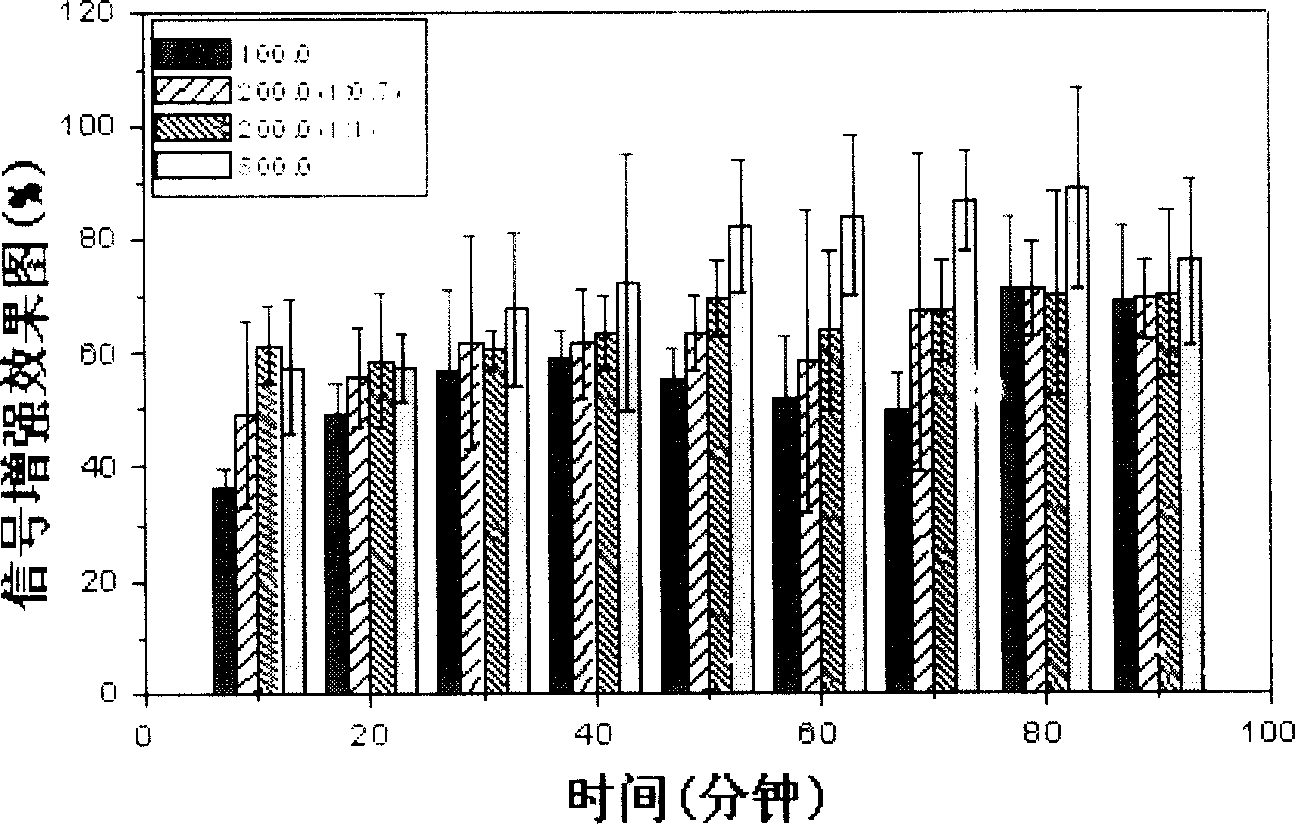
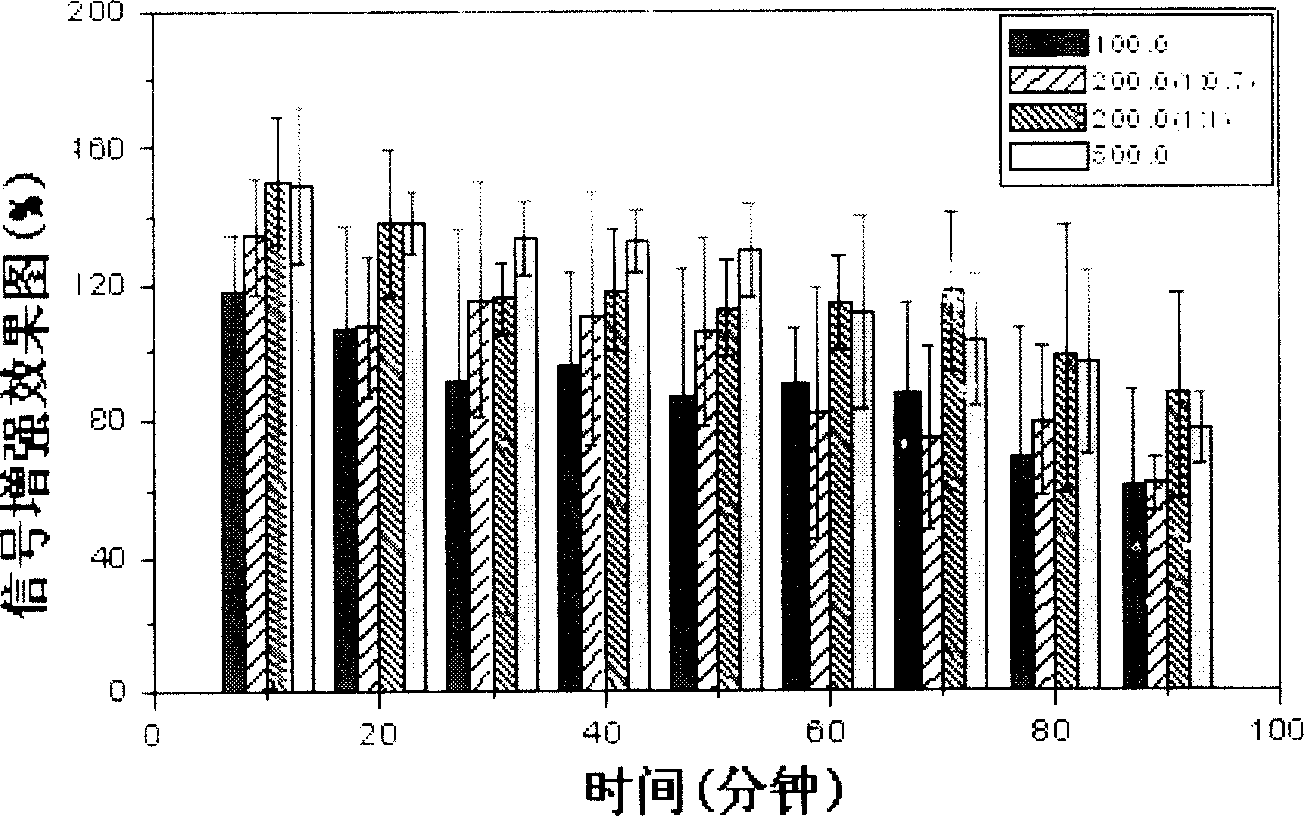
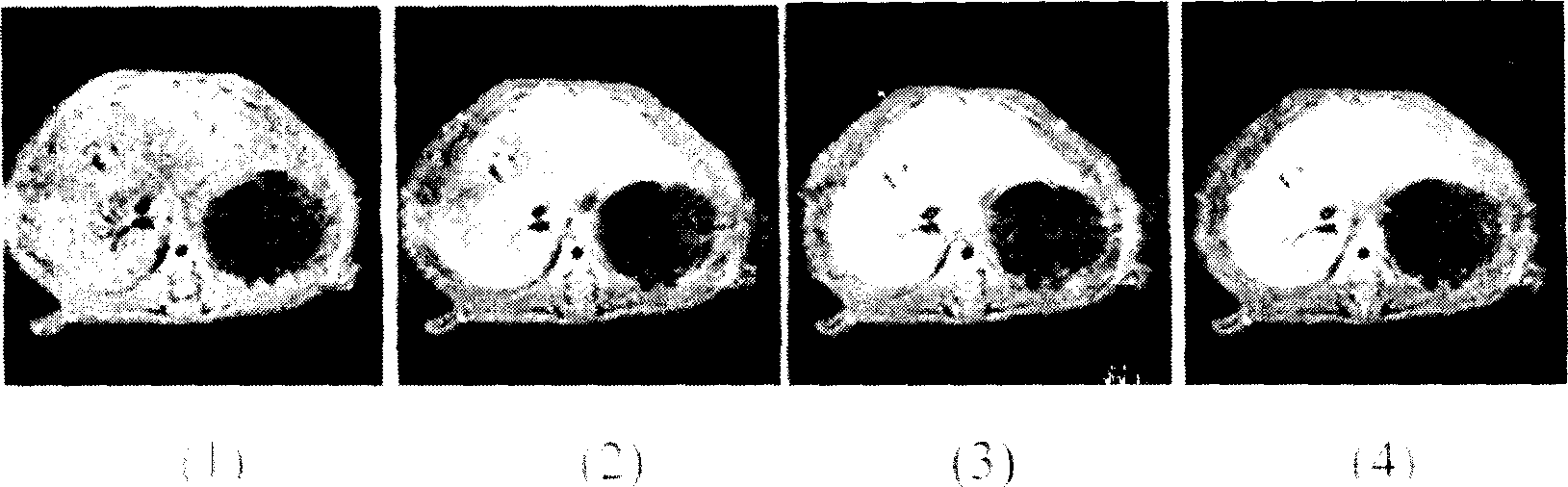
Image
Examples
Embodiment 1
[0043] Preparation of gadolinium ethylenediaminetetraacetic acid (Gd-EDTA) complexes modified with 100,000 molecular weight (100kDa) polysaccharides of Elaeagnus japonicus
[0044] (1) Preparation of ethylenediaminetetraacetic acid bis-anhydride: 36.0g of ethylenediaminetetraacetic acid (EDTA) was stirred and reacted at 64±2°C for 10 hours in 100mL of acetic anhydride and 80mL of anhydrous pyridine solution. Suction filter, wash with acetic anhydride, then with anhydrous ether, and vacuum-dry at 50°C to obtain 31.0 g of yellowish solid EDTA anhydride.
[0045] (2) Preparation of ethylenediaminetetraacetic acid modified with 100,000 molecular weight (100kDa) Elaeagnus polysaccharide: Dissolve 0.60g of EDTA anhydride in 1.00g of 100,000 molecular weight (100kDa) Elaeoptera polysaccharide in 80mL of anhydrous DMSO solution, After stirring at room temperature for 24 hours, cool in an ice-water bath, slowly add distilled water, continue to stir for 12 hours, dialyze deionized water...
Embodiment 2
[0048] Preparation of manganese ethylenediamine tetraacetate (Mn-EDTA) complexes modified with 100,000 molecular weight (100kDa) polysaccharide
[0049] (1) Preparation of ethylenediaminetetraacetic acid bis-anhydride: 36.0g of ethylenediaminetetraacetic acid (EDTA) was stirred and reacted at 64±2°C for 10 hours in 100mL of acetic anhydride and 80mL of anhydrous pyridine solution. Suction filter, wash with acetic anhydride, then with anhydrous ether, and vacuum-dry at 50°C to obtain 31.0 g of yellowish solid EDTA anhydride.
[0050] (2) Preparation of ethylenediaminetetraacetic acid modified with 100,000 molecular weight (100kDa) Elaeagnus polysaccharide: Dissolve 0.60g of EDTA anhydride in 1.00g of 100,000 molecular weight (100kDa) Elaeoptera polysaccharide in 80mL of anhydrous DMSO solution, After stirring at room temperature for 24 hours, cool in an ice-water bath, slowly add distilled water, continue to stir for 12 hours, dialyze deionized water for 5 days, change the wate...
Embodiment 3
[0053] Preparation of gadolinium diethylenetriaminepentaacetate (Gd-DTPA) complex modified with 200,000 molecular weight (200kDa) polysaccharide
[0054] (1) Preparation of diethylenetriaminepentaacetic acid bis-anhydride: put 59.0g of DTPA, 70mL of acetic anhydride and about 100mL of anhydrous pyridine into a 250mL round-bottomed flask with a condensing device at the mouth of the flask, and control the temperature at 64±2°C for 24 Hour. Suction-filtered brown-yellow insoluble matter was washed with acetic anhydride and then with anhydrous ether, and vacuum-dried at 50°C to obtain 46 g of yellowish solid DTPA anhydride.
[0055] (2) Preparation of diethylenetriaminepentaacetic acid modified with 200,000 molecular weight (200kDa) polysaccharide of Eleuthero jujube gum: Dissolve 0.70g of DTPA anhydride in 100mL of anhydrous DMSO solution of 1.00g of 200,000 molecular weight (200kDa) of polysaccharide of Elsewhere polysaccharide , after stirring at room temperature for 24 hours,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com