A class of gadolinium-containing magnetic resonance imaging contrast agent and its preparation and application
A magnetic resonance imaging and contrast agent technology, which is applied in the field of gadolinium-containing magnetic resonance contrast agents, can solve problems such as poor thermodynamic stability and dynamic stability, gadolinium ion deposition, and nephrogenic systemic fibrosis in patients, so as to improve thermodynamic and kinetic stability, damage reduction, and enhanced lipophilic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] Wherein, R is located in the ortho, meta or para position, and R represents hydrogen, halogen, alkyl, alkoxy, amine, nitro or aryl; the preparation method of a class of gadolinium-containing magnetic resonance imaging contrast agent , characterized in that the method comprises the following steps:
[0033]
[0034] (i) Add compound 1 and substituted benzaldehyde 2 in turn to the round bottom flask, then add methanol to dissolve, heat to reflux, TLC monitors the reaction process, after the reaction is over, let it stand for cooling, filter, evaporate the solvent to obtain the crude product, and recrystallize Compound 3 is obtained; the recrystallization solvent is ethyl acetate;
[0035] (ii) Under nitrogen protection, dissolve compound 3 in methylene chloride in a round-bottomed flask, then add trifluoroacetic acid, stir and react at room temperature for 24 to 48 hours, after the reaction finishes, evaporate unreacted trifluoroacetic acid under reduced pressure, Pro...
Embodiment 1
[0038] (i) Add 2mmol compound 1 and 2mmol benzaldehyde in a 100mL round bottom flask, add 30mL methanol to dissolve, heat to reflux, TLC monitors the reaction process (10% methanol / methylene chloride), after the reaction is over, let it stand for cooling, filter, evaporate The crude product was obtained by removing the solvent, and recrystallized from ethyl acetate to obtain a white solid 3a with a yield of 85%;
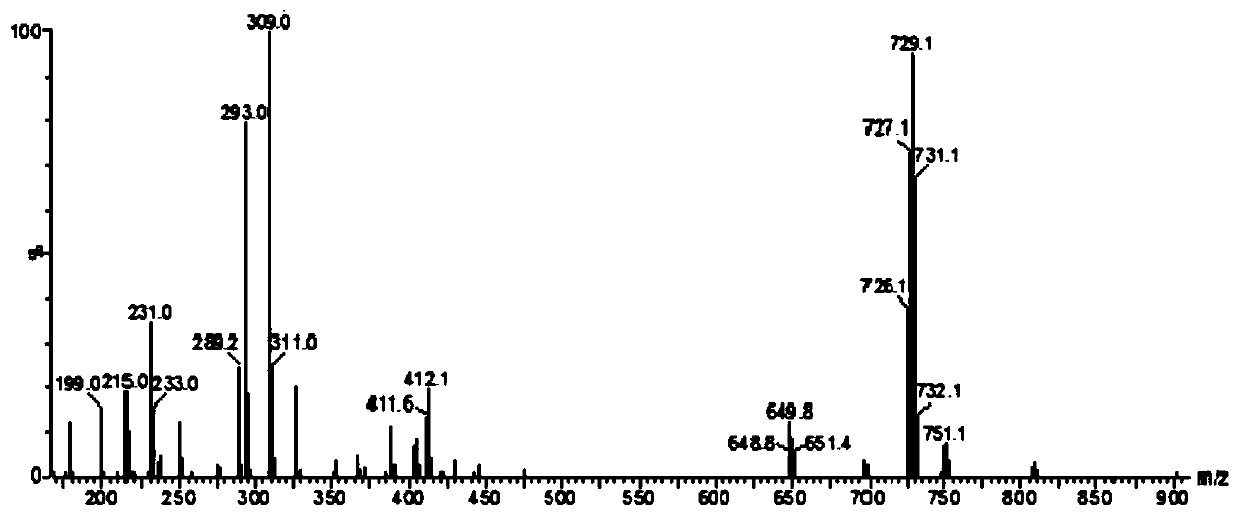
[0039] (ii) Under nitrogen protection, in a 100mL round-bottomed flask, add 1.5mmol of compound 3a and dissolve it in 10mL of dichloromethane, add 10mL of trifluoroacetic acid, stir at room temperature for 48h, after the reaction is over, distill off the unreacted trifluoro Acetic acid, recrystallized from ethyl acetate to obtain white solid 4a with a yield of 98%;
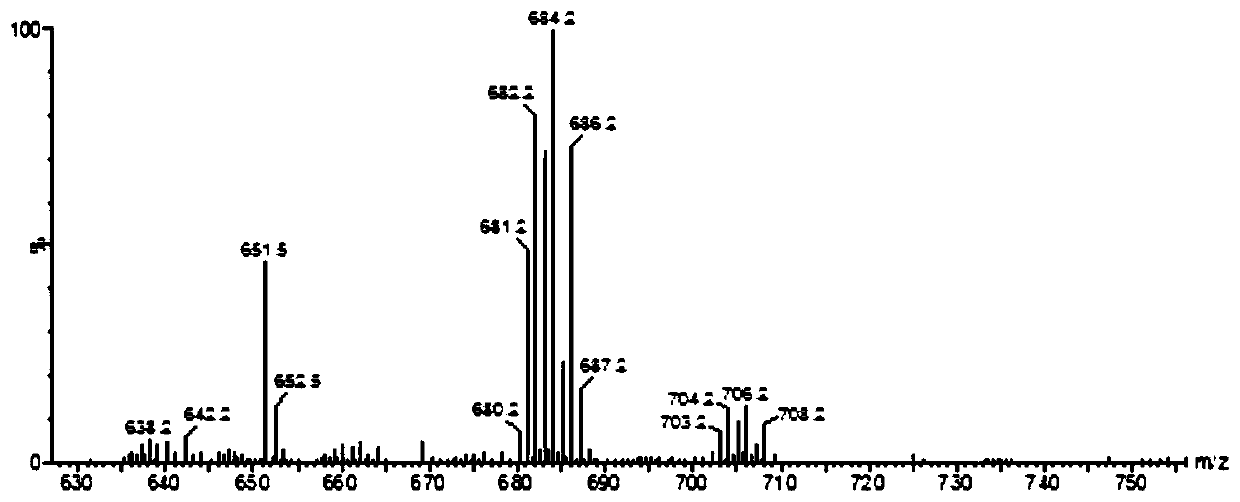
[0040] (Ⅲ) In a round bottom flask, add 1.0mmol compound 4a and dissolve it in 20mL pure water, add 0.05M GdCl 3 22mL aqueous solution, adjust the pH value of the solution to 6.5 with 1M NaOH, react at ...
Embodiment 2
[0042] (i) Add 2mmol compound 1 and 2mmol m-nitrobenzaldehyde in a 100mL round-bottomed flask, add 30mL methanol to dissolve, heat to reflux, TLC monitors the reaction process (10% methanol / methylene chloride), after the reaction finishes, let it stand for cooling, Filtration, evaporation of the solvent to obtain a crude product, recrystallization from ethyl acetate to obtain a white solid 3b, yield 90%;
[0043] (ii) Under nitrogen protection, in a 100mL round bottom flask, add 1.5mmol of compound 3b and dissolve it in 10mL of dichloromethane, add 10mL of trifluoroacetic acid, stir at room temperature for 24h, after the reaction is over, distill off unreacted trifluoroacetic acid under reduced pressure Acetic acid, recrystallized from ethyl acetate to obtain white solid 4b with a yield of 99%;
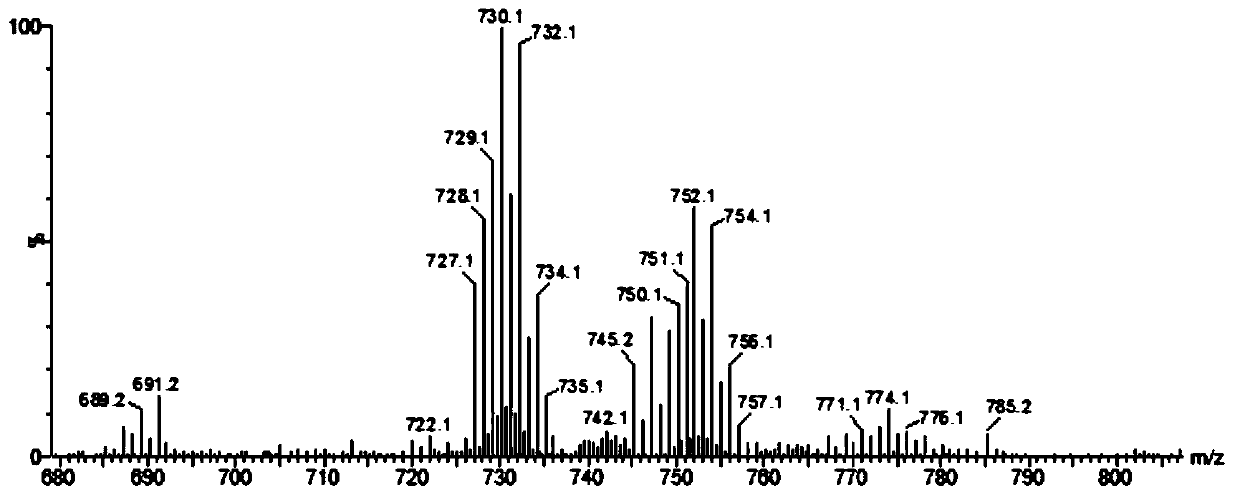
[0044] (Ⅲ) In a round bottom flask, add 1.0mmol compound 4b and dissolve it in 20mL pure water, add 0.05M GdCl 322mL aqueous solution, adjust the pH value of the solution to 7.0 with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com