Method of preparing nano-zinc borate
A nano-zinc borate and nano-technology, which is applied in the direction of borate, boron oxide, etc., can solve the problem of nano-particle size difference, and achieve the effect of improving interface binding force and easy dispersibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
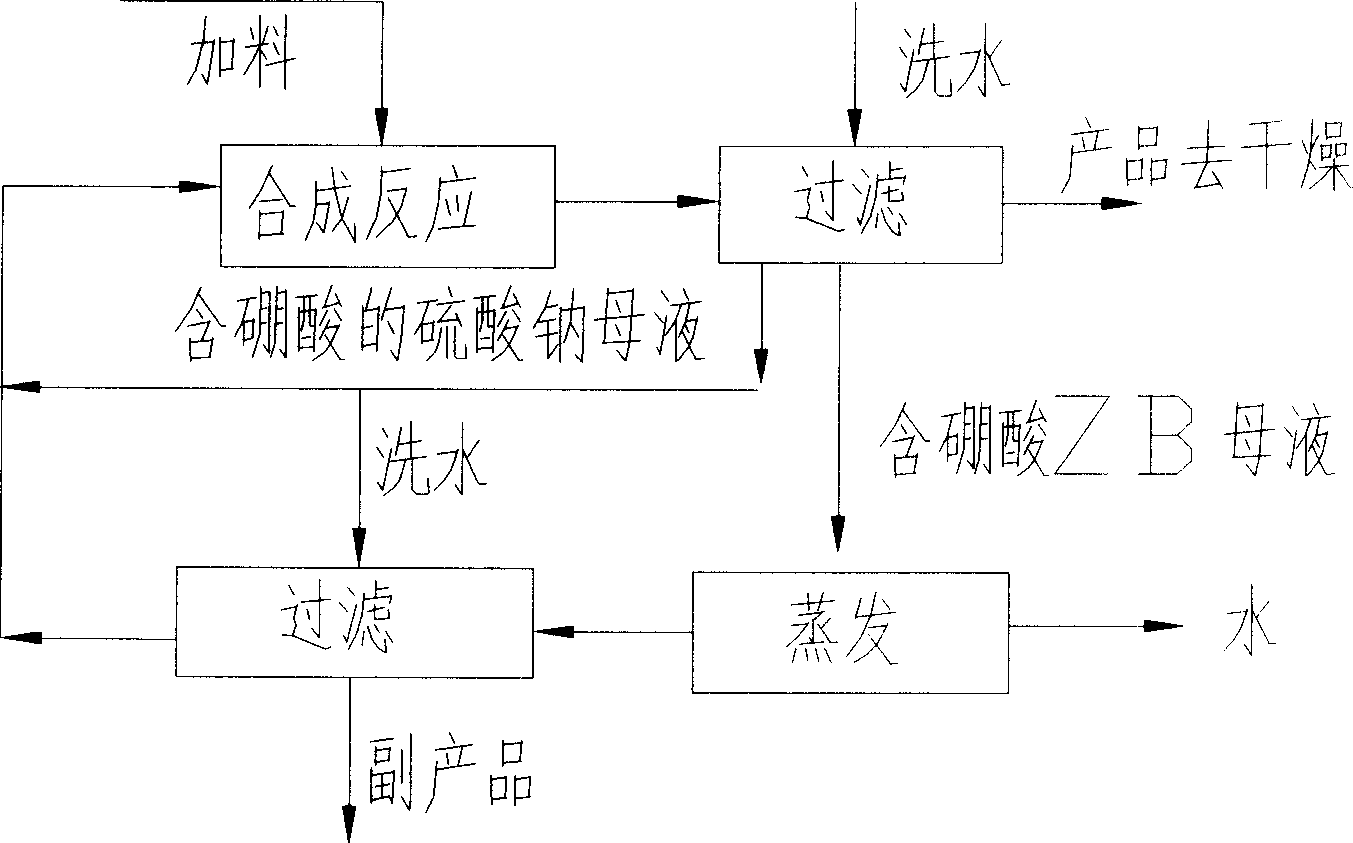
[0050]Example 1: take by weighing 3.24Kg borax respectively, after 3.24Kg zinc sulfate and 0.0324Kg zinc oxide are mixed, add 4.56Kg water and 0.15Kg N-propyltrimethoxysilane, modulate into rheological state, change to airtight container at 80 ℃ for 3 hours, keep the concentration of boric acid at 0.6 mol / l during the reaction process, after cooling to room temperature, take out the reaction product, filter, and dry at 120-140 ℃ to obtain the desired nano-zinc borate product. as attached figure 1 Shown flow process, the sodium sulfate mother liquor in last production process returns in the synthesis reactor, and reacts together with the newly added zinc sulfate, borax, zinc oxide. After the reaction is complete, let it stand for half an hour, filter while it is hot, and send the obtained nano-zinc borate into a dry box to obtain a finished product. The mother liquor is sent to the evaporator, evaporated to the saturation point of sodium sulfate and boric acid, and sodium sulf...
example 2
[0053] Weigh 7.00Kg of borax, 5.00Kg of zinc sulfate and 0.08Kg of zinc oxide and mix them, then add 7.5Kg of water and 0.12Kg of N-propyltrimethoxysilane to make a rheological state, transfer them to a closed container and react at 100°C for 3 hours During the reaction process, the concentration of boric acid is kept at 0.3 mol / l. After cooling to room temperature, the reaction product is taken out, filtered, and dried at 120-140° C. to obtain the desired nano-zinc borate product.
example 3
[0055] Weigh 1.0Kg of borax, 5.0Kg of zinc sulfate and 0.08Kg of zinc oxide and mix them, then add 1.82Kg of water and 0.12Kg of N-propyltriethoxysilane to make a rheological metamorphosis, transfer them into a closed container and react at 100°C for 5 Keep the concentration of boric acid at 0.4 mol / l during the reaction process. After cooling to room temperature, take out the reaction product, filter, and dry at 120-140° C. to obtain the desired nano-zinc borate product.
PUM
| Property | Measurement | Unit |
|---|---|---|
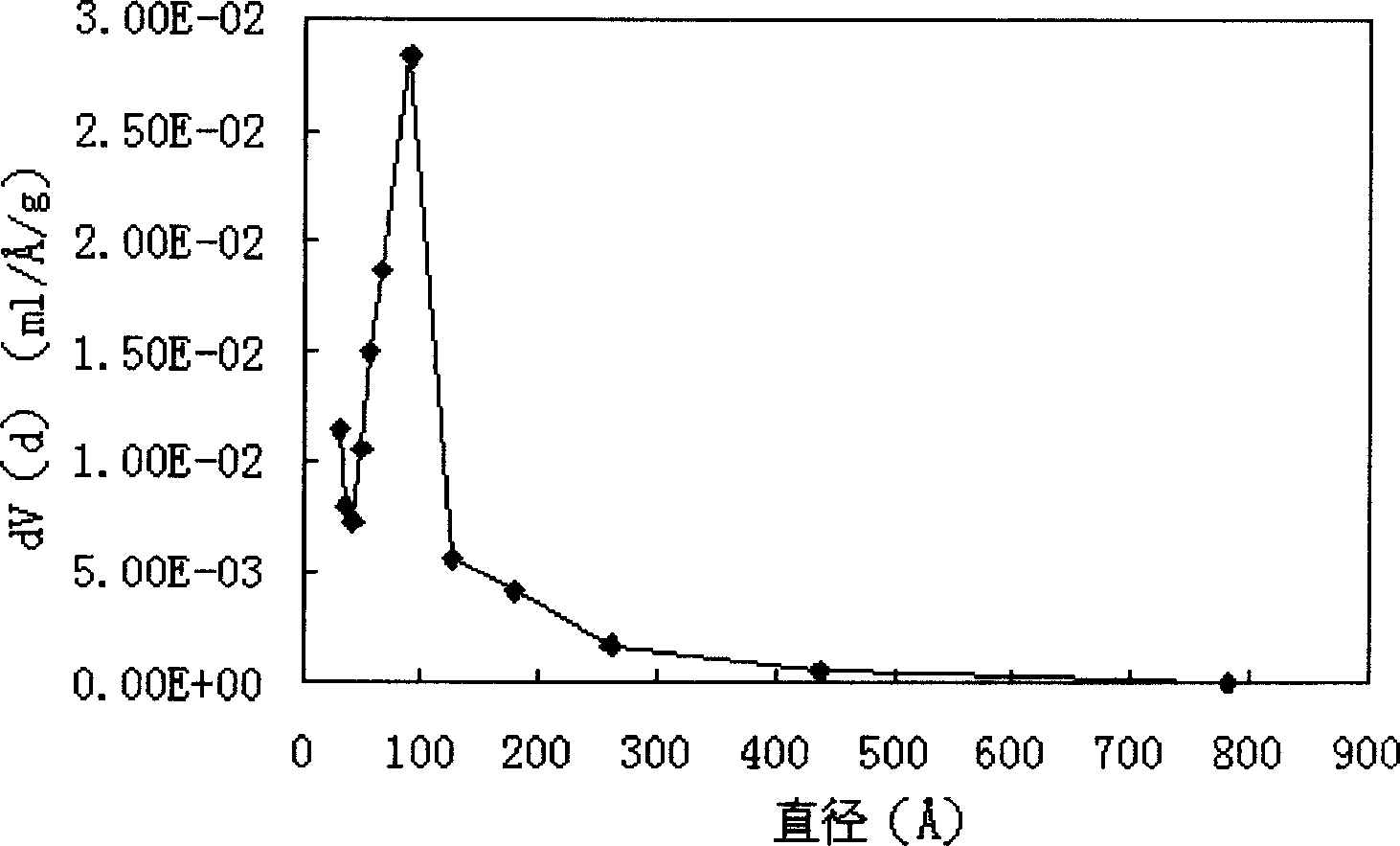
| Average pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com