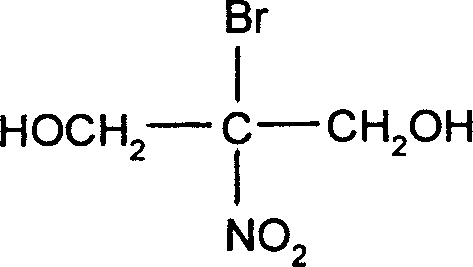
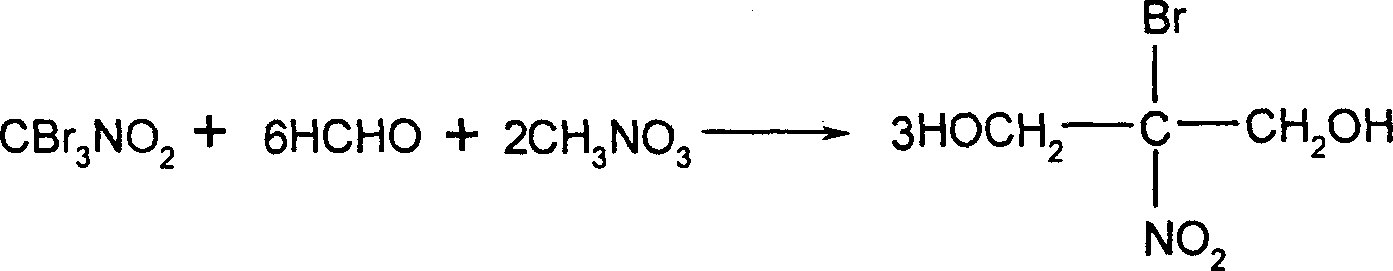
Preparation method of 2-bromo-2-nitro-1,3-propylene glycol
A technology of propylene glycol and tribromonitromethane, applied in the field of 2-bromo-2-nitro-1, can solve the problems of increased impurities, low utilization rate of bromine, reduced content, etc., and achieves reduction of production cost, realization of cleaning, The effect of increased utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the preparation method of 2-bromo-2-nitro-1,3-propanediol
[0032] In a 1-liter reactor, put 1 mole of nitromethane, 50 milliliters of water, and 1.5 moles of bromine, slowly add 3 moles of 30% sodium hydroxide solution and stir for 1 hour, and feed 110 grams of chlorine gas at 30 to 40 ℃, stirred and reacted for 1 hour, and stood to separate layers to separate 310.8 g of tribromopicrin with a purity of 99% and a yield of 98%. 310.8 grams of the bromopicrin reaction solution obtained by the above reaction, 180 grams of formaldehyde, and 131 grams of nitromethane were stirred and reacted for 1 hour. Below 40 ° C, 10% sodium hydroxide solution was slowly added dropwise, and at 30 React at ~40°C for 1 hour, cool, crystallize, filter, and dry to obtain 564.2 g of bronopol finished product with a purity of 99% and a yield of 95%.
Embodiment 2
[0033] Embodiment 2: the preparation method of 2-bromo-2-nitro-1,3-propanediol
[0034] In a 1-liter reactor, put 1 mole of nitromethane, 50 milliliters of water, and 1.5 moles of bromine, slowly add 3 moles of 30% sodium hydroxide solution and stir for 1 hour, and feed 106.5 grams of chlorine gas at 30 to 40 ℃, stirred and reacted for 1 hour, stood to separate layers, and separated 306.7 g of tribromopicrin with a purity of 99% and a yield of 96.7%. 306.7 grams of the bromopicrin reaction solution obtained by the above reaction, 180 grams of formaldehyde, and 131 grams of nitromethane were stirred and reacted for 1 hour. Below 40 ° C, 10% sodium hydroxide solution was slowly added dropwise, and at 30 ~ React at 40°C for 1 hour, cool, crystallize, filter, and dry to obtain 562.6 g of bronopol finished product with a purity of 99% and a yield of 96%.
Embodiment 3
[0035] Embodiment 3: the preparation method of 2-bromo-2-nitro-1,3-propanediol
[0036] In a 1-liter reactor, put 1 mole of nitromethane, 50 milliliters of water, and 1.5 moles of bromine, slowly add 3 moles of 30% sodium hydroxide solution and stir for 1 hour, and feed 110 grams of chlorine gas at 30 to 40 ℃, stirred and reacted for 1 hour, and stood to separate layers to separate 310.8 g of tribromopicrin with a purity of 99% and a yield of 98%. 310.8 grams of the bromopicrin reaction solution obtained by the above reaction, 180 grams of formaldehyde, and 131 grams of nitromethane were stirred and reacted for 1 hour. Below 40 ° C, 10% potassium hydroxide solution was slowly added dropwise. React at 40°C for 1 hour, cool, crystallize, filter, and dry to obtain 569.7 g of bronopol finished product with a purity of 99% and a yield of 94%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com