Method of preparing chloropy gra hydrogen sulphate type I
A technology of clopidogrel hydrogen sulfate and clopidogrel salt, which is applied in the field of preparation of type I clopidogrel hydrogen sulfate, can solve the problem that the crystal form purity can only be controlled at 98%, and achieve the effect of increasing the crystallization speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
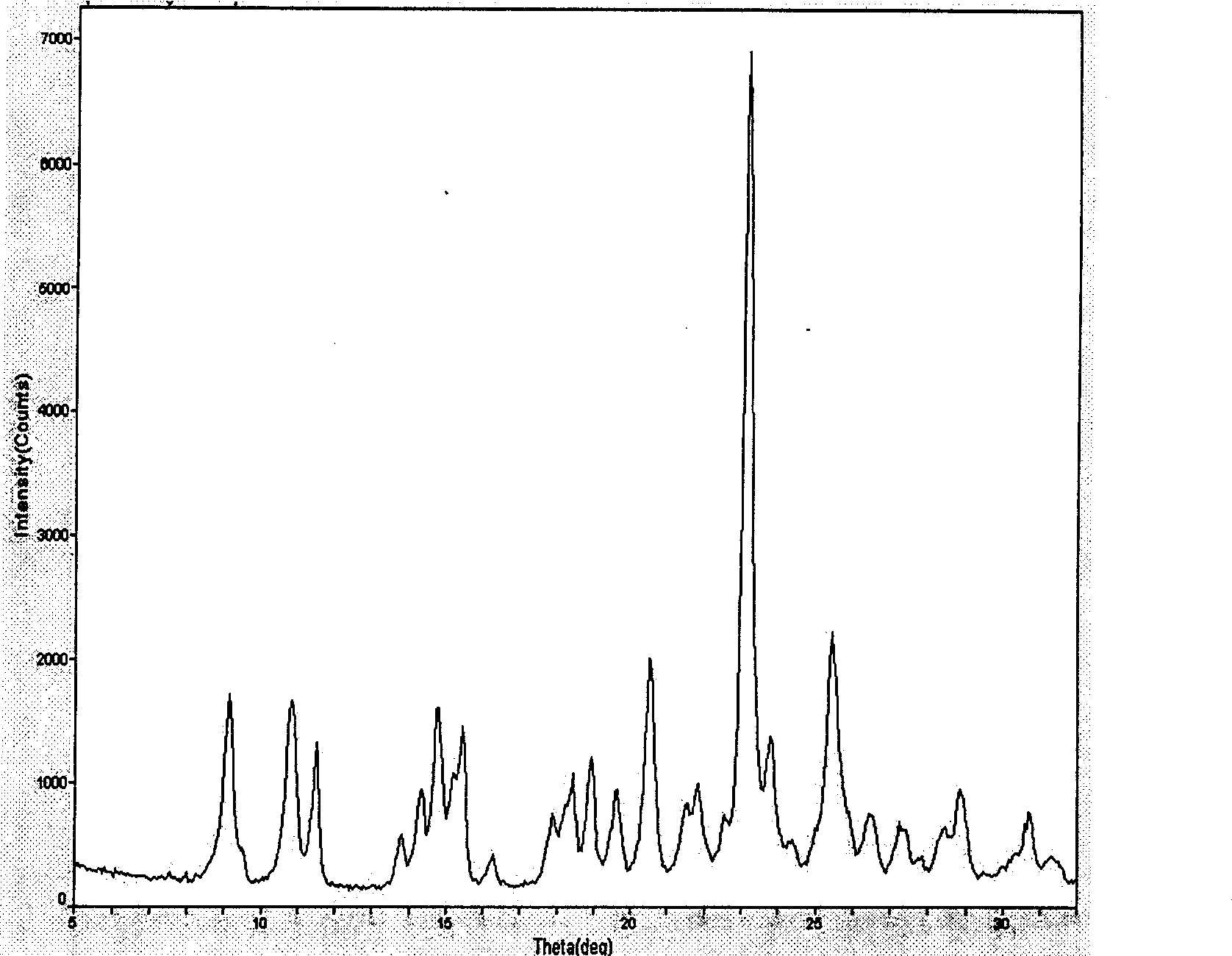
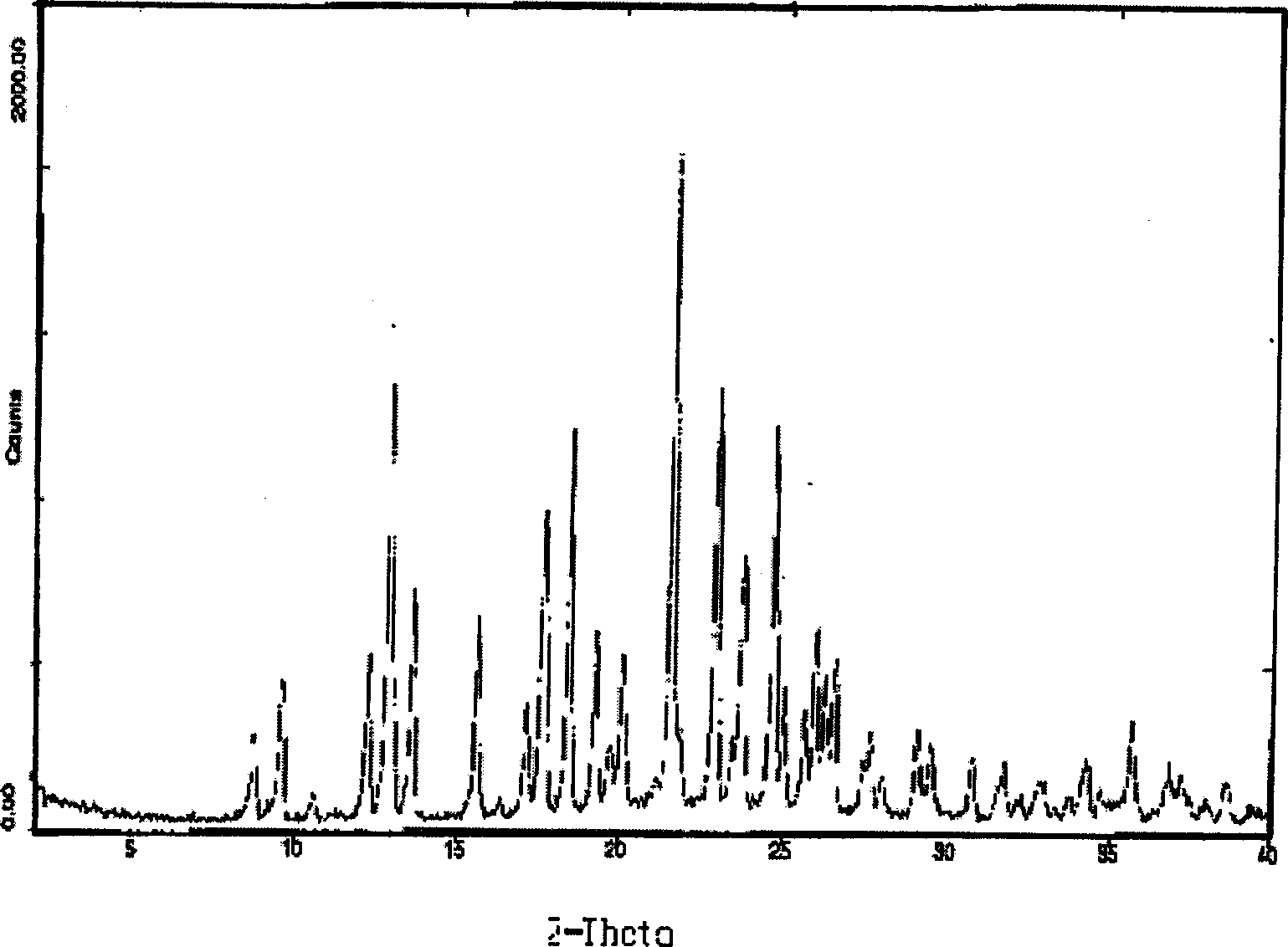
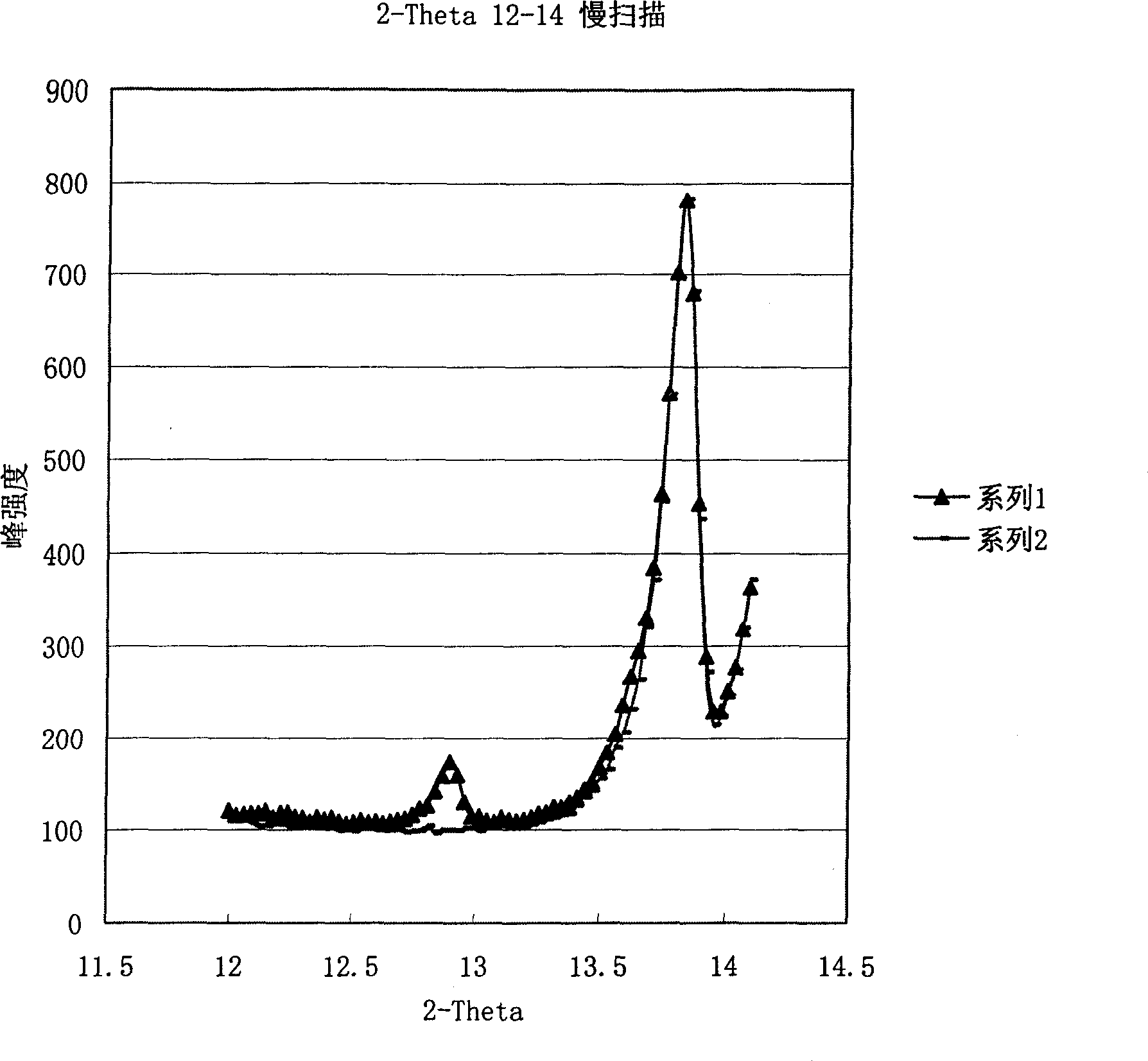
[0025]Under nitrogen protection, 7.93 grams (0.222mol) of clopidogrel hydrochloride and 100 milliliters of dichloromethane were added to a 250 milliliter three-necked flask, and under stirring and cooling with ice water, 3.2 grams of potassium carbonate and 50 milliliters of water were added dropwise to form The solution. Stir for one hour, and the pH value of the upper layer should be greater than 8. The layers were separated after standing, and the lower organic phase was separated; the upper aqueous layer was extracted once with 50 ml of dichloromethane. The organic phases were combined, and dichloromethane was recovered to dryness. Add 50 ml of 2-pentanone to the free base and stir for one hour to completely dissolve the free base. Cool to -5-0°C, add 0.2 g of clopidogrel type I bisulfate to the system, start to drop 1.96 g of sulfuric acid (0.200 mol), and control the internal temperature between -5-0°C during the dropping. After the dropwise addition, the temperature ...
example 2
[0027] Add 320 milliliters of 3-pentanone into a three-necked flask with 321 grams (1.0 mol) of clopidogrel free base, cool to 0-5°C, add 5 grams of clopidogrel hydrogensulfate seed crystals of type I, and then dropwise add 93.1 grams of concentrated sulfuric acid (0.95mol), the internal temperature is controlled at 0-5°C during the dropwise addition, and the dropwise addition is completed within 1 hour. After the sulfuric acid was added dropwise, 2 grams of clopidogrel type I sulfate hydrogen sulfate seed crystals were added again, and the temperature was raised to 40-50° C. at a rate of 20° C. per hour, and kept stirring at 40-50° C. for 30 minutes. Suction filtration under reduced pressure and washing with a small amount of 3-pentanone to obtain 358 g of the product with a yield of 85%. The melting point of the product is 181 degrees, and its specific rotation is: 55.7° (c=1, methanol). It is a type I product detected by infrared and X-diffraction, and the crystal form puri...
Embodiment 3
[0029] Under nitrogen protection, 5.56Kg (10mol) polymorphic clopidogrel camphorsulfonate and 100 liters of dichloroethane were added to a 250-liter reactor, and 2.0Kg of sodium carbonate was added dropwise under stirring and cooling with ice water. Make an aqueous solution with 50 liters of water. Stir for one hour, the pH value of the upper layer should be greater than 7.5, let stand to separate the layers, and separate the lower organic phase; the upper aqueous layer is extracted once with 50 liters of dichloroethane. The organic phases were combined, and dichloroethane was recovered to dryness. Add 50L of tert-butyl methyl ketone to the free base, stir for one hour to completely dissolve the free base, cool to -10~-5°C, add dropwise 9.8Kg of 10% sulfuric acid tert-butyl methyl ketone that has been cooled below -10°C Solution, control the internal temperature during the dropwise addition between -10 and 5°C. After the dropwise addition, 50 g of clopidogrel type I seed cry...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Specific rotation | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com