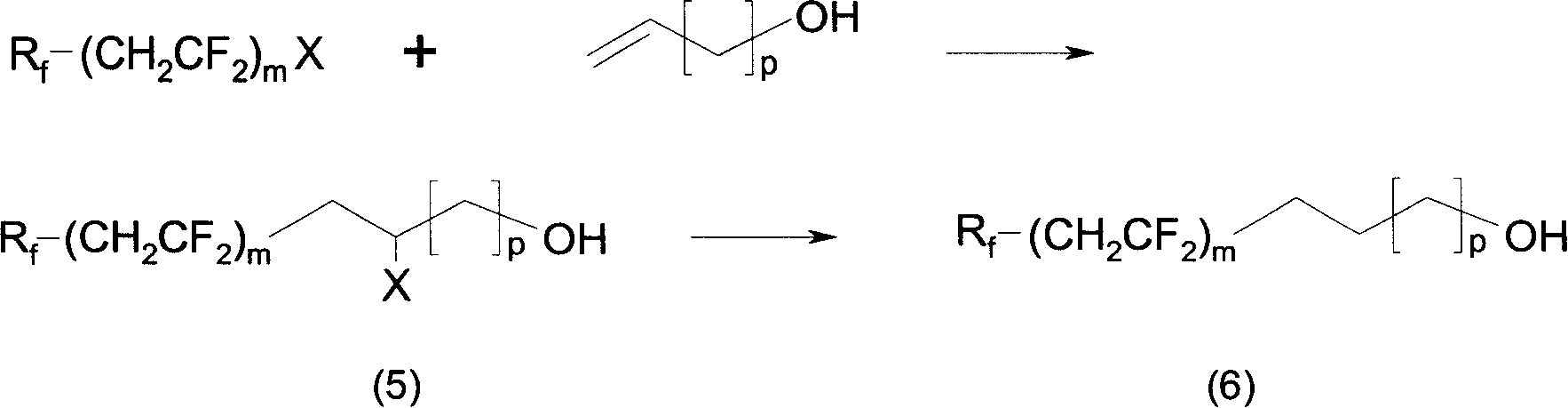
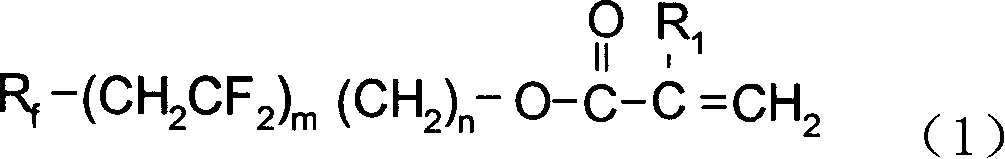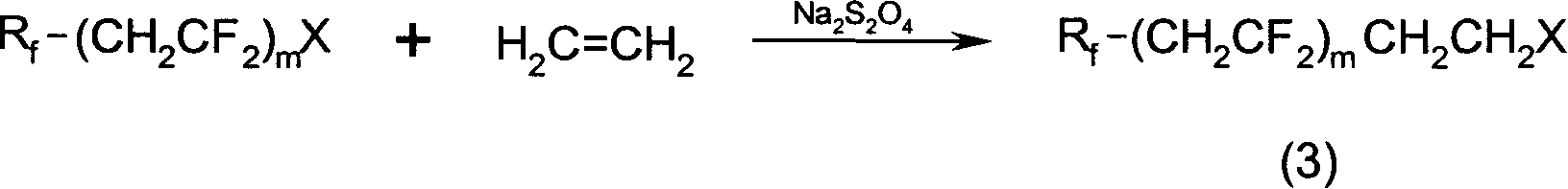Fluorine-containing carbon chain acrylate, preparation method and application for copolymer thereof
A carbon chain acrylate and copolymer technology, applied in textiles and papermaking, devices for coating liquids on surfaces, coatings, etc. Water repellent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Synthesis of Fluorinated Acrylate Copolymer Using Perfluoroiodohexane as Starting Material
[0048] In a 100ml autoclave, add 10 grams of perfluoroiodohexane, 0.1 grams of cuprous chloride, and 5.43 grams of vinylidene fluoride. Heated to 300°C and reacted for 48 hours. After the reaction was completed, the autoclave was cooled in an ice-water bath to release unreacted vinylidene fluoride gas. Purification gives 1-iodo-2H, 2H-perfluorooctane.
[0049] The relevant data are as follows: 1 H NMR (300MHz, CDCl 3 )δ3.42(m, J=15.6, 2H); 19 FNMR (282MHz, CDCl 3 ( s, 2F), -126.17(m, 2F).
[0050] In a 100ml autoclave, add 27.87 grams of 1-iodo-2H, 2H-perfluorooctane, 0.975 grams of sodium dithionite, 70 milliliters of diglyl dimethyl ether, and feed ethylene gas, react for 8 hours, and find the pressure in the kettle Decrease, stop the reaction, release excess ethylene gas, and distill to obtain 1-iodo-1H, 1H, 2H, 2H, 4H, 4H-perfluorodecane.
[0051] The relevant data ...
Embodiment 2
[0060] Synthesis of Fluorinated Acrylate Copolymer Using Perfluoroiodobutane as Starting Material
[0061] In a 0.1-liter autoclave, add 100 grams of perfluoroiodobutane and 32 grams of vinylidene fluoride. Heated to 300°C and reacted overnight. After the reaction was complete, the autoclave was cooled in an ice-water bath to release the remaining gas. Purification affords pure 1-iodo-2H,2H,4H,4H-perfluorooctane.
[0062] The relevant data are as follows: 1 H NMR (300MHz, CDCl 3 )δ3.41(m, 2H), 2.83(m, 2H); 19 F NMR (282MHz, CDCl 3 )δ -38.82 (m, 2F), -81.08 (m, 3F), -88.10 (m, 2F), -112.60 (m, 2F), -124.42 (s, 2F), -125.87 (m, 2F).
[0063] In a 250 ml three-necked flask, 5.57 g of Na 2 S 2 o 4 Added to 9.48 g of 1-iodo-2H,2H,4H,4H-perfluorooctane, 3.8 g of vinyl ether, and 150 ml of tetrahydrofuran. After 15 hours of reaction, C 4 f 9 CH 2 CF 2 CH 2 CF 2 CH 2 CHO without further purification.
[0064] The above product was dissolved in 40 ml of butanol, and a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com