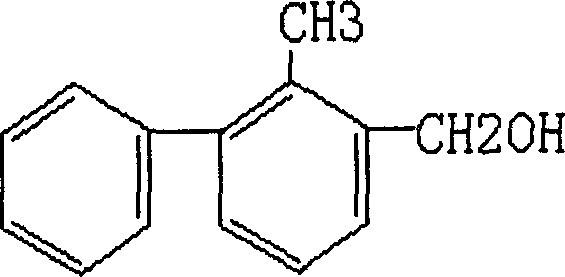
Method for preparing 2-methyl-3-phenyl benzil alcohol
A technology of phenyl benzyl alcohol and methyl biphenyl is applied in the field of preparation of 2-methyl-3-phenyl benzyl alcohol, can solve the problems of low activity, low process yield, low product content and the like, and achieves the yield High performance, good product purity and convenient process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific example 1
[0035] Add 130g of magnesium chips into a 2000ml four-necked bottle, then add 1000g of tetrahydrofuran and stir, add 0.65g of iodine to initiate the reaction, then raise the temperature to reflux and add 750g of 2,6-dichlorotoluene dropwise, the dropping temperature is kept at reflux, and the dropwise addition is completed Afterwards, reflux and keep warm for 1 hour, and set aside.
[0036] Add 750g of bromobenzene, 50g of tetrahydrofuran and 2g of activated carbon loaded with a metal nickel halide compound into a 2000ml four-necked flask, raise the temperature to reflux, add the above reaction solution dropwise, and reflux for 2 hours after the dripping is completed. The above reaction solution was poured into 5% dilute sulfuric acid solution for hydrolysis, and the hydrolysis temperature was controlled at 30°C. Heating up to the kettle temperature of 100°C for precipitation, stopping, cooling down to normal temperature, and stratifying. After the lower water layer was separ...
specific example 2
[0040] Add 260g of magnesium chips into a 5000ml four-necked bottle, then add 2200g of tetrahydrofuran and stir. After adding 1.5g of iodine to trigger, the temperature is raised to reflux and 1400g of 2,6-dichlorotoluene is added dropwise. The dropping temperature is kept at reflux. After the dropwise addition is completed Reflux for 1 hour and set aside.
[0041] Add 1400g of benzene bromide, 110g of tetrahydrofuran and 3.5g of activated carbon loaded with a metal nickel halide compound into a 5000ml four-neck flask, raise the temperature to reflux, add the above reaction solution dropwise, and reflux for 2 hours after the dripping is completed. The above reaction solution was poured into 5% dilute sulfuric acid solution for hydrolysis, and the hydrolysis temperature was controlled at 30°C. Heating up to the kettle temperature of 100°C for precipitation, stopping, cooling down to normal temperature, and stratifying. After the lower water layer was separated, the oil layer w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com