Liquid-phase method for synthesizing organo-silicon ether
A liquid-phase synthesis, organohydrogensilane technology, applied in the direction of organic chemistry, chemical instruments and methods, compounds of elements of group 4/14 of the periodic table, etc., to achieve the effect of less reaction steps, easy realization, and environmental friendly cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
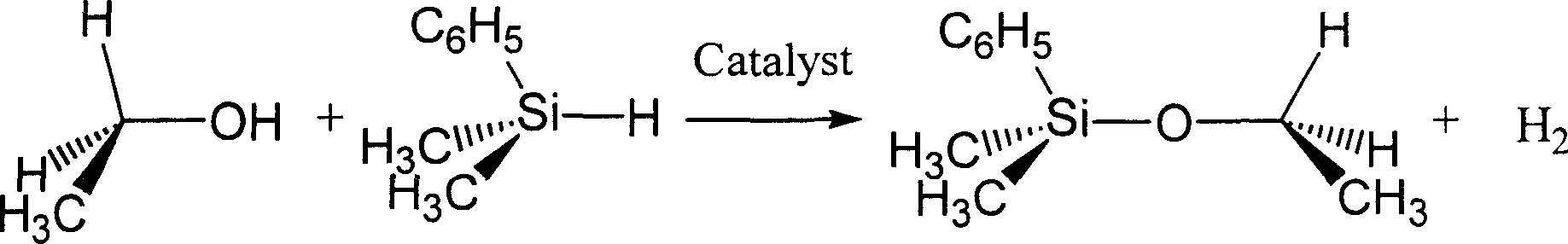
[0020] Embodiment 1: Ethyl, dimethylphenyl silyl ether C 2 h 5 OSi(CH 3 ) 2 (C 6 h 5 )Synthesis
[0021]
[0022] Under anhydrous and oxygen-free conditions, accurately weigh dimethylphenylhydrosilane (CH 3 ) 2 (C 6 h 5 ) SiH 1.440 g is 10.5 mmol and benzoyl manganese pentacarbonyl C 6 h 5 COMn(CO) 5 0.030 g is 0.1 mmol, and accurately weighed into anhydrous ethanol C in another 100 ml round flask B 2 h 5 0.460 g of OH is 10.0 mmol and about 30 ml of dehydrated benzene C is added 6 h 6 .
[0023]Use rubber sealing plugs to plug the two bottle mouths tightly, take them out and fix them on the iron stand, and use metal binding wires to further fasten the rubber sealing plugs. Connect the rubber tube with an oil-sealed glass bubbling pressure relief bypass with inert gas through the injection needle to the flask A equipped with a magnetic rotor, and turn on the magnetic stirrer. Pierce the rubber sealing plugs of the two bottles of A and B with two pointed al...
Embodiment 2
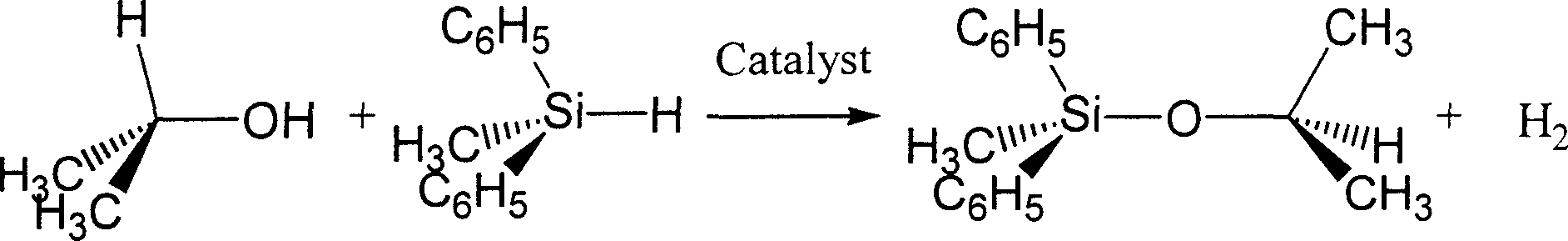
[0026] Embodiment 2: Isopropyl, methyl diphenyl silyl ether (CH 3 ) 2 CHOSi (CH 3 )(C 6 h 5 ) 2 Synthesis
[0027]
[0028] Under anhydrous and oxygen-free conditions, accurately weigh methyldiphenylhydrosilane (CH 3 )(C 6 h 5 ) 2 SiH 2.080 g is 10.5 mmol and pentacarbonyl manganese bromide BrMn(CO) 5 0.027 grams is 0.1 mmol. Accurately weigh anhydrous isopropanol (CH 3 ) 2 CHOH 0.600 g is 10.0 mmol and add about 30 ml of dehydrated benzene C 6 h 6 .
[0029] Use rubber sealing plugs to plug the two bottle mouths tightly, take them out and fix them on the iron stand, and use metal binding wires to further fasten the rubber sealing plugs. Connect the rubber tube with an oil-sealed glass bubbling pressure relief bypass with inert gas through the injection needle to the flask A equipped with a magnetic rotor, and turn on the magnetic stirrer. Pierce the rubber sealing plugs of bottle A and bottle B with alloy hollow hard wires with pointed tips at both ends, s...
Embodiment 3
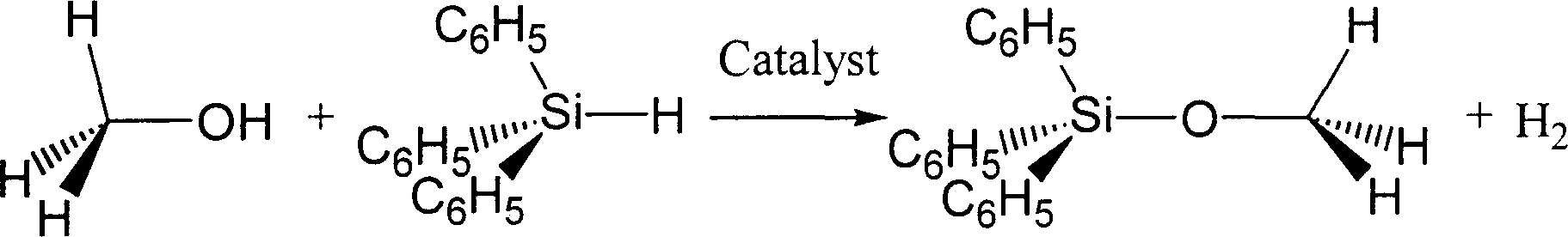
[0032] Example 3: methyl, triphenylsilyl ether CH 3 OSi(C 6 h 5 ) 3 Synthesis
[0033]
[0034] Under anhydrous and oxygen-free conditions, accurately weigh triphenylhydrosilane (C 6 h 5 ) 3 SiH 2.600 g i.e. 10.0 mmol and methyl manganese pentacarbonyl CH 3 Mn(CO) 5 0.021 grams is 0.1 mmol. In another 100 ml round flask B, accurately weigh anhydrous methanol CH 3 0.340 g of OH is 10.5 mmol and about 30 ml of dehydrated benzene C is added 6 h 6 .
[0035] Use rubber sealing plugs to plug the two bottle mouths tightly, take them out and fix them on the iron stand, and use metal binding wires to further fasten the rubber sealing plugs. Connect the rubber tube with an oil-sealed glass bubbling pressure relief bypass with inert gas through the injection needle to the flask A equipped with a magnetic rotor, and turn on the magnetic stirrer. Pierce the rubber sealing plugs of bottle A and bottle B with alloy hollow hard wires with pointed tips at both ends, so that t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com