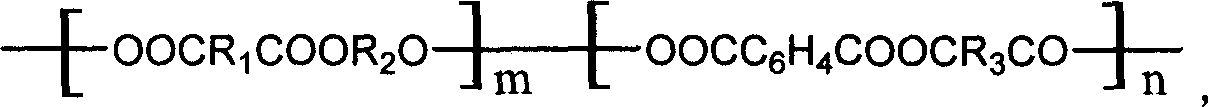Biodegradable high-molecular-weight aliphatic/aromatic copolymer, and its preparing method and use
A biodegradable, high-molecular-weight technology, applied in the field of biodegradable high-molecular-weight aliphatic/aromatic copolymers, can solve the problems of difficult adjustment of biodegradation rate, low glass transition temperature, and polybutylene succinate crystallinity advanced questions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032]The molecular weight and molecular weight distribution of a biodegradable high molecular weight aliphatic / aromatic copolymer obtained according to the preparation method provided by the present invention are measured by gel permeation chromatography (GPC). Polystyrene with a series of molecular weights with a narrow molecular weight distribution is used as a calibration standard sample, chloroform is used as an eluting phase, and the measurement temperature is 40°C.
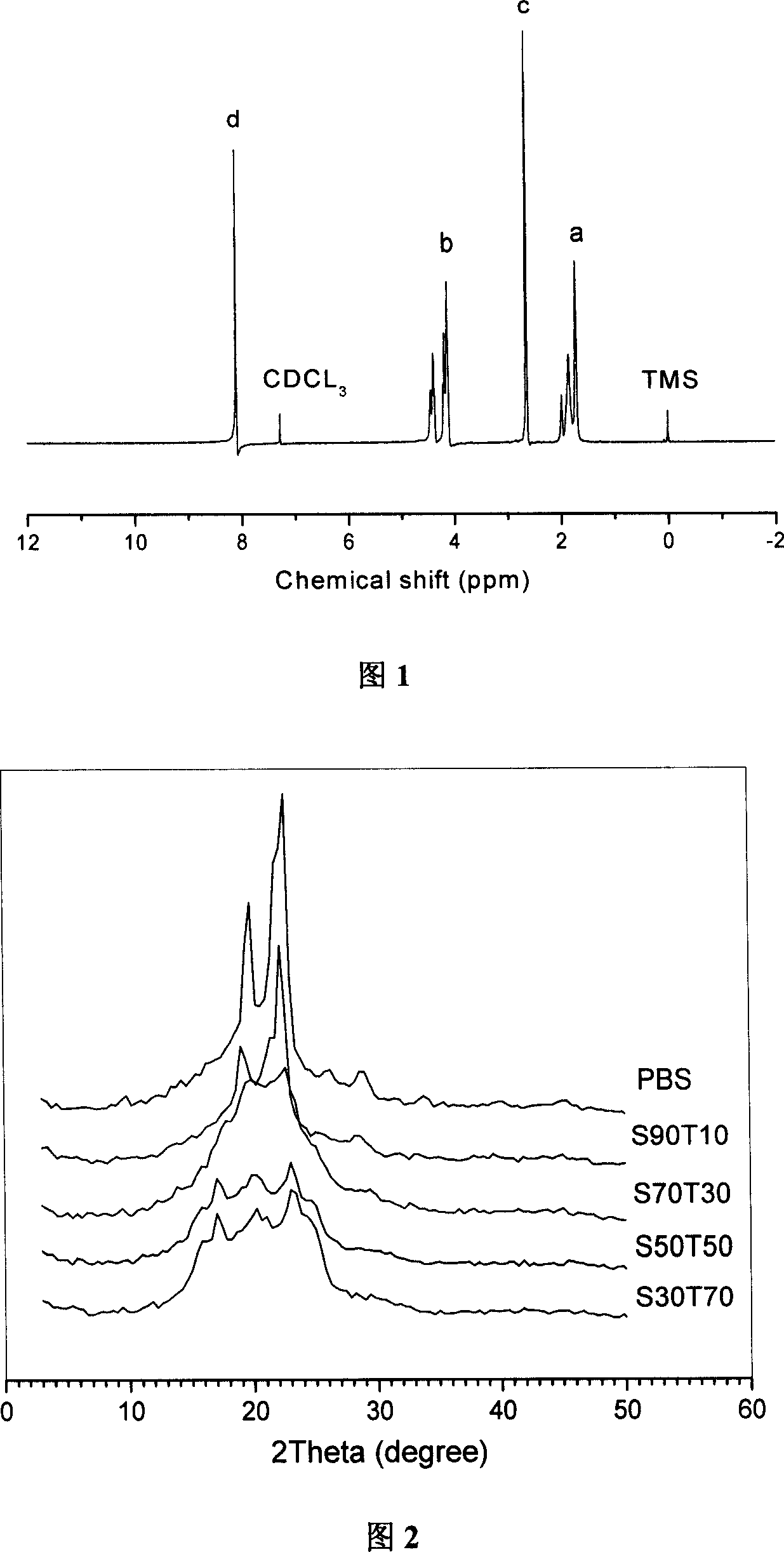
[0033] 【Chemical Composition and Structure】
[0034] The structure and composition of the compound obtained according to the preparation method provided by the present invention are determined by nuclear magnetic resonance (NMR) of the solution, the measurement temperature of the NMR is room temperature, and the solvent is deuterated chloroform.
[0035] 【Thermophysical properties】
[0036] By differential scanning calorimetry (DSC), the melting point and glass transition temperature of the compound can be...
Embodiment 1
[0040] At room temperature, 26.28g of dimethyl succinate, 3.88g of dimethyl terephthalate, 18.9g of 1,4-butanediol and 56.8mg of tetraisopropoxytitanium were sequentially added to a 200ml volume four of high-purity nitrogen replacement. necked round bottom flask. At the same time, the four-necked flask is equipped with a high-purity nitrogen gas inlet, a mechanical stirrer, and a condensation water separator. The above reaction system was moved into an oil bath at 180°C, stirred and reacted for 1 to 2 hours with nitrogen gas, and then decompressed slowly, and the temperature of the oil bath was gradually increased to 230°C. The vacuum degree is controlled within 150Pa. When the torque value of the stirring rod is constant or the product is entangled on the stirring rod and forms a ball, the reaction is considered to be over. After cooling, the product of the above polymerization reaction was first dissolved in chloroform, and then filtered, and excess cold methanol was added...
Embodiment 2
[0042] At room temperature, 20.44g of dimethyl succinate, 11.64g of dimethyl terephthalate, 18.9g of 1,4-butanediol and 18.9mg of tetraisopropoxytitanium were sequentially added to a 200ml volume four of high-purity nitrogen replacement. necked round bottom flask. At the same time, the four-neck flask is equipped with a high-purity nitrogen gas inlet, a mechanical stirrer, and a condensation water separator. The above reaction system was moved into an oil bath at 180°C, stirred and reacted for 1 to 2 hours with nitrogen gas, and then decompressed slowly, and the temperature of the oil bath was gradually increased to 230°C. The vacuum degree is controlled within 150Pa. When the torque value of the stirring rod is constant or the product is entangled on the stirring rod and forms a ball, the reaction is considered to be over. After cooling, the product of the above polymerization reaction was first dissolved in chloroform, and then filtered, and excess cold methanol was added ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Thermal decomposition temperature | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com