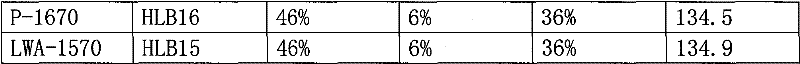Wuji Baifeng dispersing tablet
A technology of black-bone chicken, white phoenix, and dispersible tablets, which is applied to medical preparations containing active ingredients, plant/algae/fungus/moss ingredients, pill delivery, etc. It can solve problems such as difficult industrialization, adhesion, and dissolution rate, etc. Achieve the effect of large room for maneuver and improved disintegration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Chinese medicine raw materials of black-bone chicken and Baifeng dispersible tablets: 750 grams of black-bone chicken (hair removal, claws, intestines), 280 grams of astragalus (honey roast), 375 grams of rehmannia glutinosa, 280 grams of codonopsis, 95 grams of Chuanxiong, 188 grams of angelica, white peony root (wine Fried) 188 grams, Schisandra (wine) 95 grams, yam 560 grams, Atractylodes macrocephala 280 grams, Poria cocos 188 grams, Moutan bark 188 grams.
[0058] The weight percentage of traditional Chinese medicine extract and auxiliary materials of Wuji Baifeng Dispersible Tablets: 20% fine powder of Wuji Baifeng extract, 25% low-substituted hydroxypropyl cellulose, 19% sodium carboxymethyl starch, 10% microcrystalline cellulose , lactose 20%, polyvinylpyrrolidone 3%, magnesium stearate 3%.
[0059] Black-bone Chicken Baifeng Dispersible Tablets are obtained according to the following specific steps: the above twelve flavors, the yam is crushed into fine powder;...
Embodiment 2
[0062] 28% by weight of fine powder of Wuji Baifeng extract, 22% sodium carboxymethylcellulose, 16% cross-linked polyvinylpyrrolidone, 12% microcrystalline cellulose, 5% polyvinylpyrrolidone, 10% lactose, polyethylene Pyrrolidone 4%, micronized silica gel 1%, magnesium lauryl sulfate 2%. All the other are with embodiment 1.
Embodiment 3
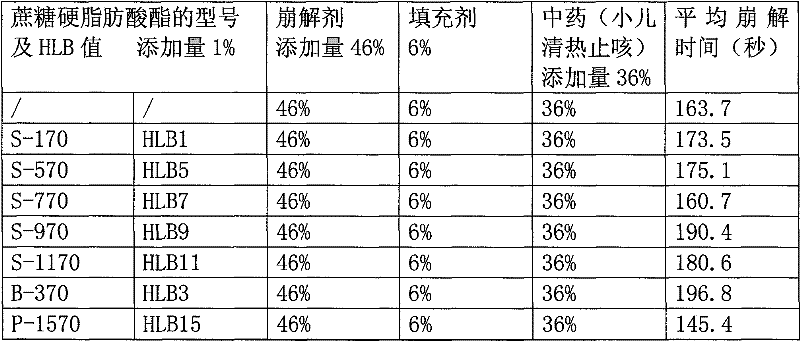
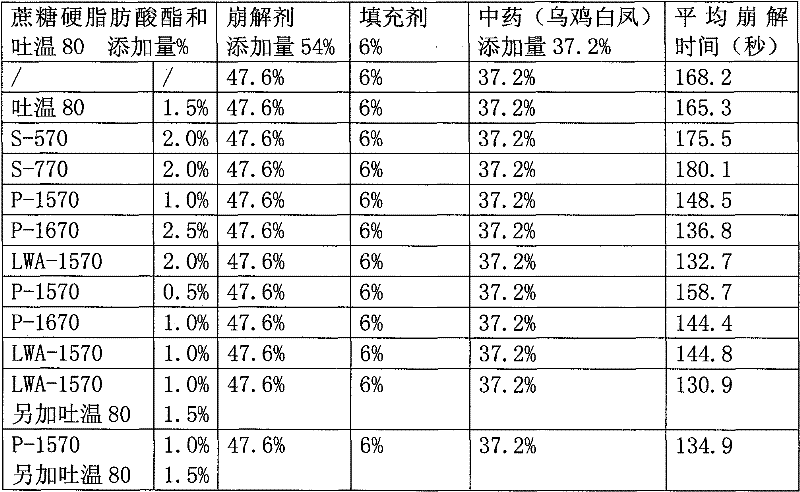
[0064] 36% by weight of fine powder of Wuji Baifeng extract, 20% sodium carboxymethyl starch, 15% croscarmellose sodium, 6% crospovidone, 3% low-substituted hydroxypropyl cellulose , mannitol 9%, polyvinylpyrrolidone 7%, micronized silica gel 2%, magnesium stearate 1%, HLB=15-16 sucrose fatty acid ester 1%. Sucrose fatty acid ester is P-1570 or P-1670 or LWA-1570, all the other are the same as embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com