Low-toxic and broad-spectrum phthalocyanine bactericide, its production and use
A low-toxicity, bactericide technology, applied in the field of preparation of new phthalocyanine photosensitizers, can solve the problem of bacterial resistance to oral microenvironment damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
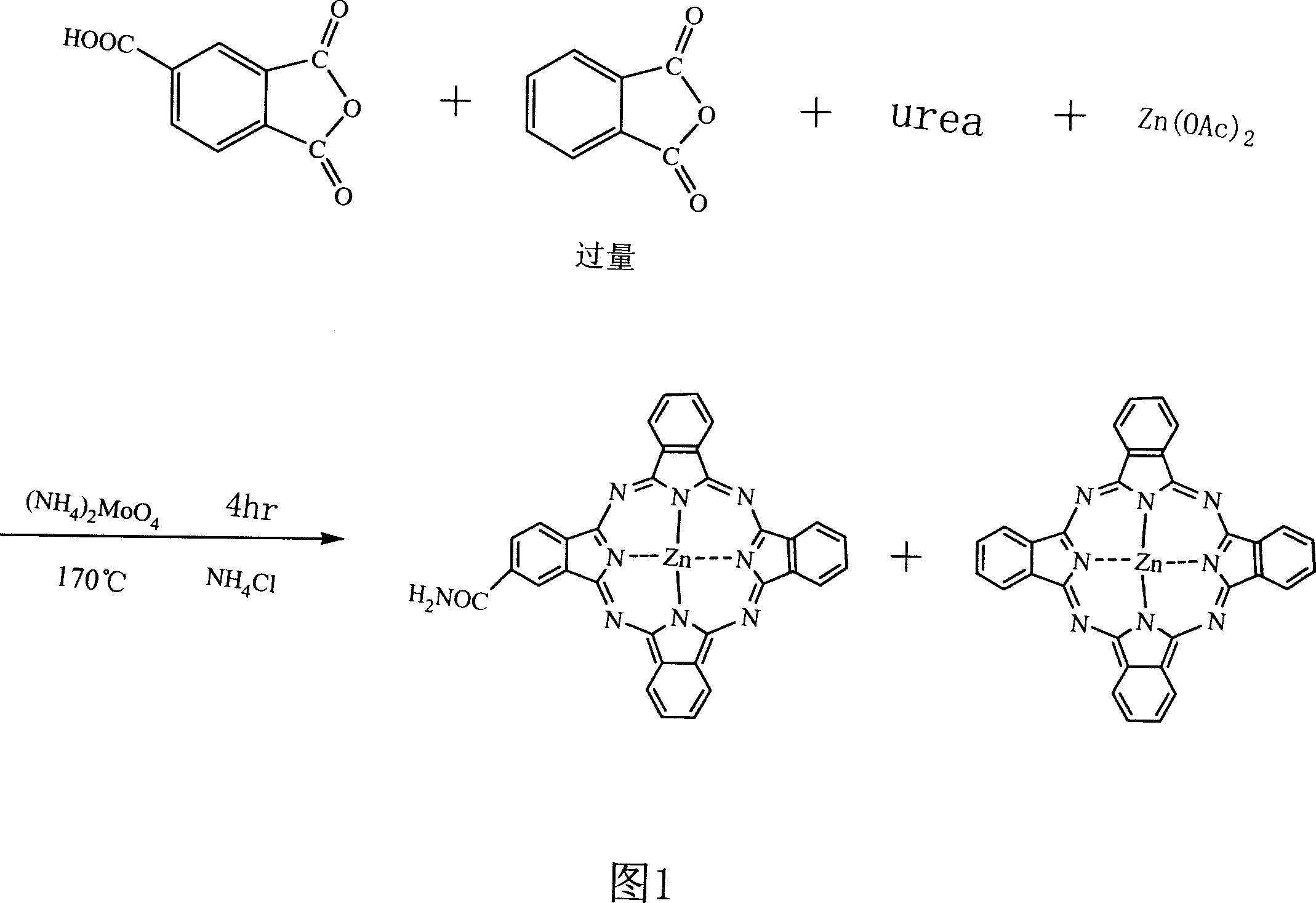
[0017] Synthesis of embodiment one 2-monoamide substituted zinc phthalocyanine
[0018] Add 2.40g (0.0125mol, Merck) of trimellitic anhydride, 12.96g (0.08750mol) of phthalic anhydride, and 22.00g of anhydrous zinc acetate into a 500ml three-necked flask with a reflux device and a stirring bar. (0.1002mol), urea 60g (1mol), ammonium molybdate 0.50g (0.4mmol), ammonium chloride 2.00g (0.0374mol). Heated to 170°C in an oil bath and reacted for 4 hours. After the reaction was completed, it was washed several times with 0.5M HCl, and then washed with pure water to neutrality to obtain 22.25 g of solid. The yield was 67%, 22% of which was 2-monoamide substituted zinc phthalocyanine as judged by HPLC.
Embodiment 2
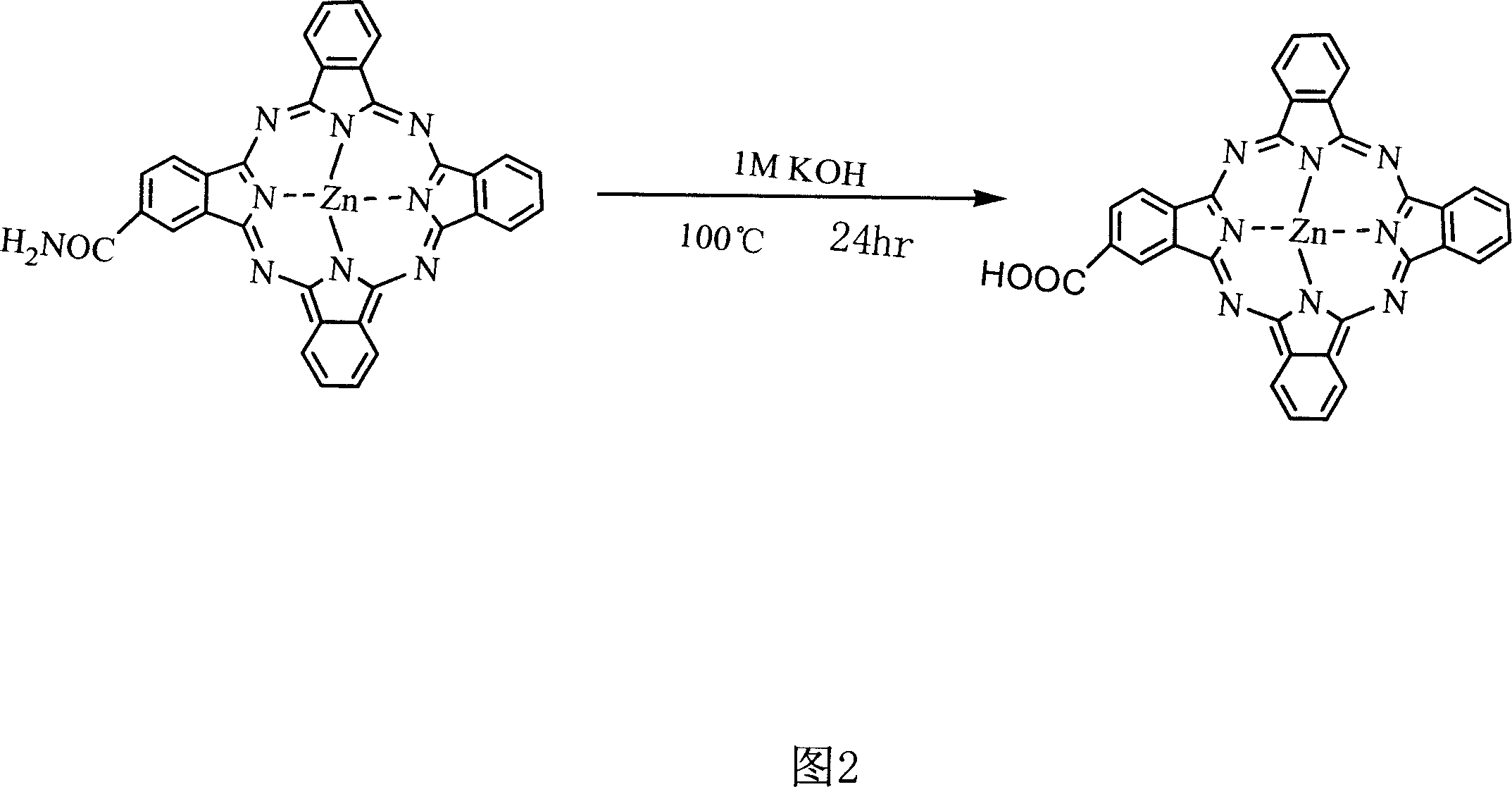
[0019] Synthesis of embodiment two 2-monocarboxy substituted zinc phthalocyanines
[0020] Take 15.00 g of the 2-monoamide-substituted zinc phthalocyanine prepared in Example 1 into a 250 ml three-necked bottle, add 100 ml of 1M KOH, and stir and reflux at 100° C. for 24 hours. Suction filtration after the reaction was completed to obtain a green solid, which was washed by adding 0.5M KOH and suction filtration until the filtrate was colorless. Then wash with 1M HCl several times, and then wash with water until neutral drying.
Embodiment 3
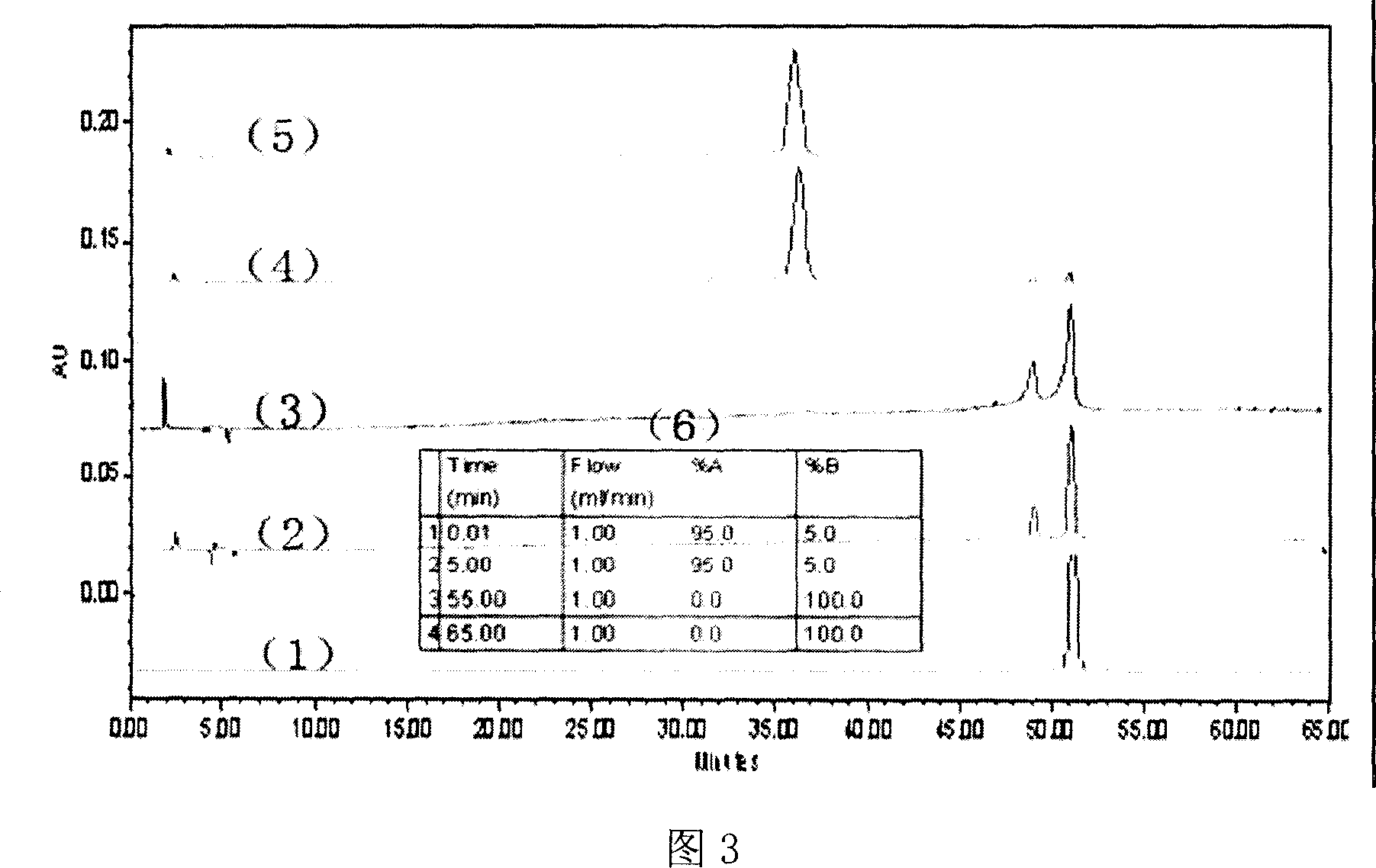
[0021] Example 3 Separation and purification of 2-monocarboxy substituted zinc phthalocyanine
[0022]Select a chromatographic column with a length of 40 cm and an internal diameter of 3 cm, use 100 mesh silica gel as the adsorbent, use a mixed solvent of DMF: acetone = 3: 1 as the eluent, and use a mixed solvent of DMF: acetone = 3: 1 for wet packing , the column height is 30cm. 1.85 g of the crude product after the reaction treatment in the previous step was dissolved in 37 ml of DMF. The sample volume is 1ml each time, and the column can be used repeatedly. During the elution process, the blue-green phthalocyanine sample was found to be divided into two fractions, and the second fraction (the one eluted later) was collected. The collected samples were freeze-dried. The dried sample was subjected to column separation again according to the above method, and the unsubstituted zinc phthalocyanine and the 2-monoamide substituted zinc phthalocyanine could be removed by repeat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com