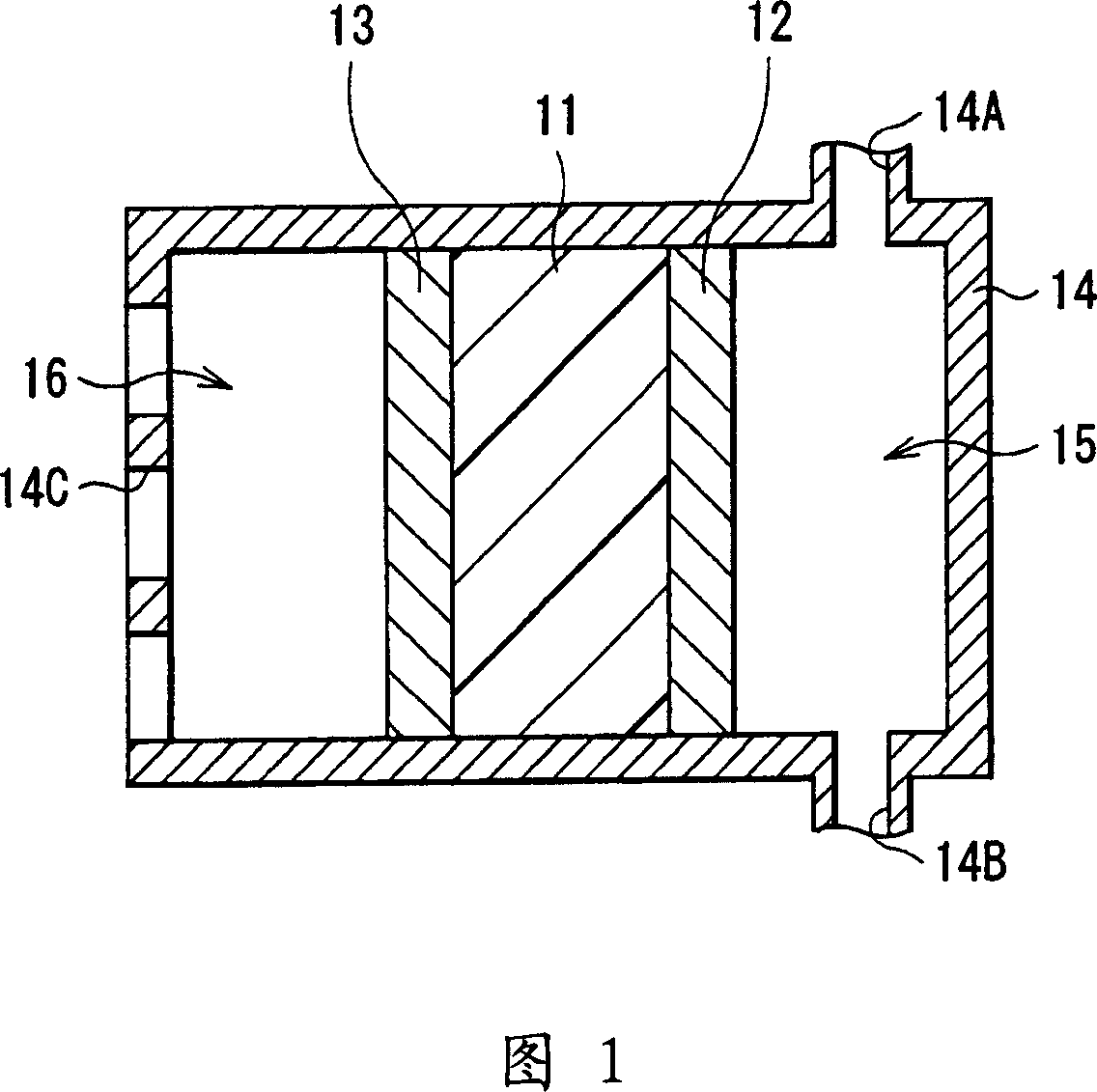
Mixture, cation conductor and electrochemical device using those
A technology of mixtures and cations, applied in the direction of conductive materials, conductive materials, organic material conductors, etc., can solve the problems of long-term continuous use difficulties, the decline of proton conductivity, etc., and achieve the effect of preventing the reduction and preventing the conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1 to 1-6
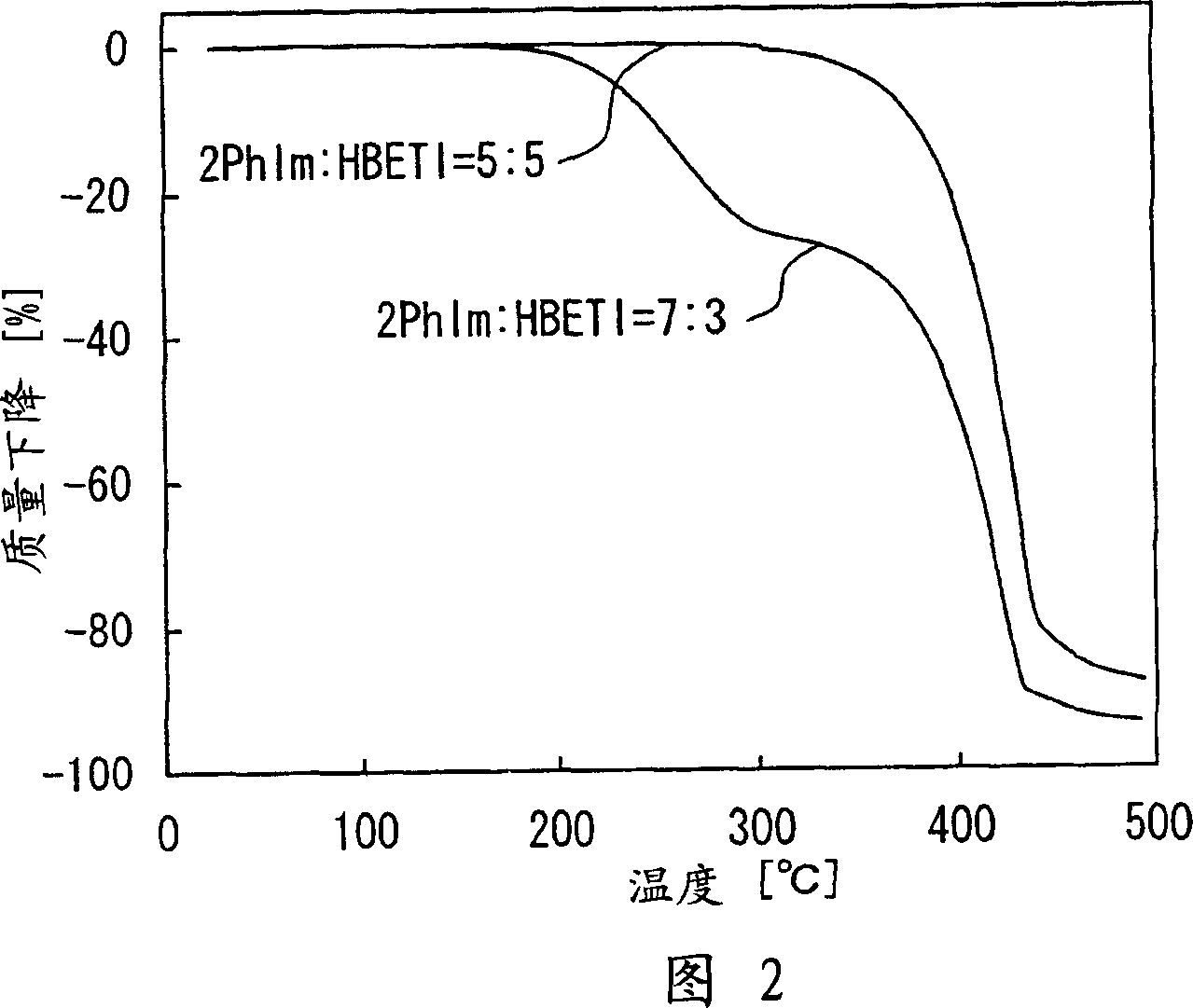
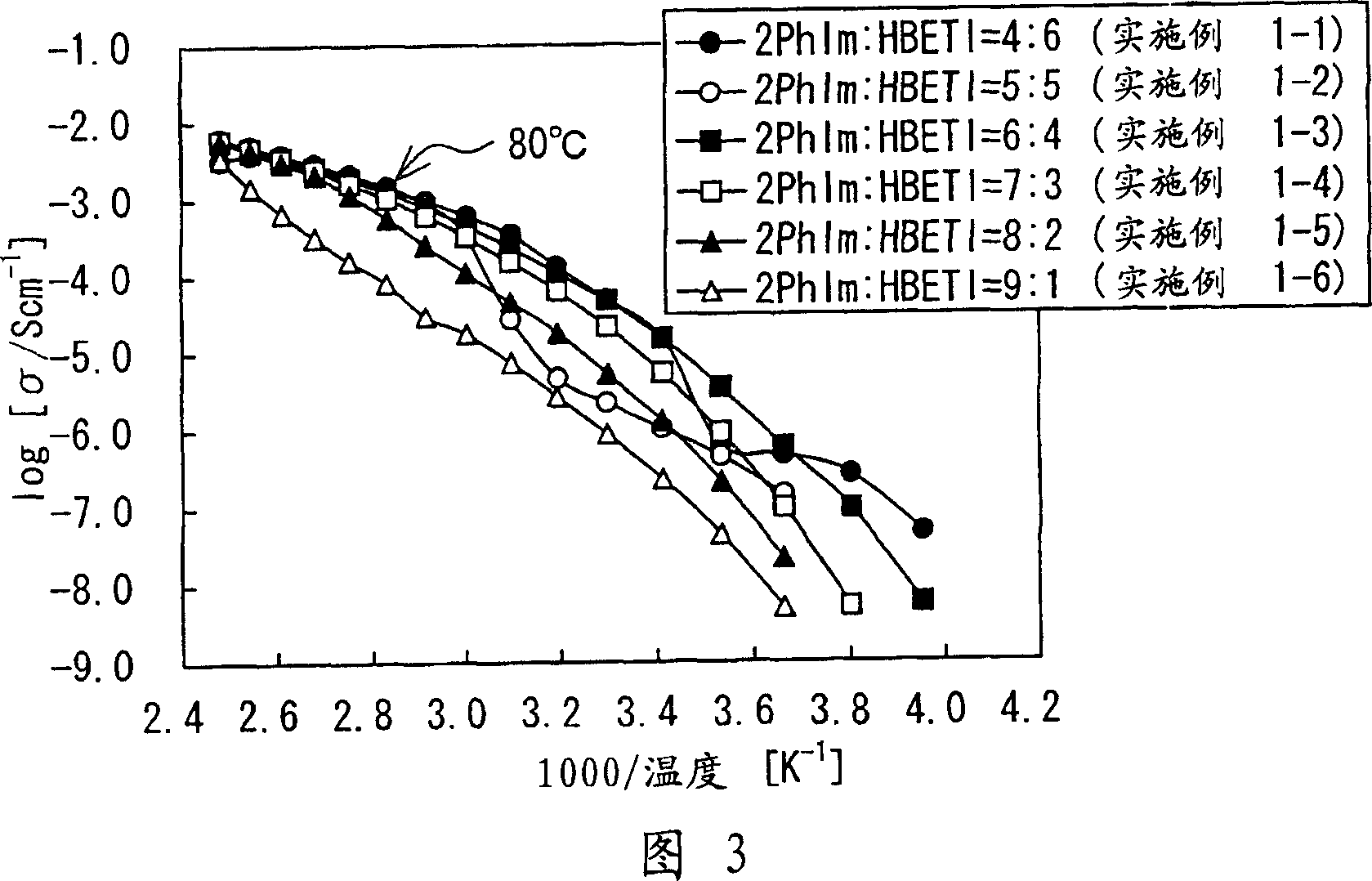
[0127] The mixture described in this embodiment is formed. For the first compound, 2-phenylimidazole represented by Chemical Formula 21 (hereinafter referred to as "2PhIm") was used. As the second compound, pentafluoroethanesulfonimido (hereinafter referred to as "HBETI") represented by Chemical Formula 26 was used. In Examples 1-1 to 1-6, the molar ratio between 2PhIm and HBETI in the mixture was changed to 2PhIm:HBETI=4:6, 5:5, 6:4, 7:3, 8:2 or 9 : 1. Specifically, relative to 1.524g (4.00mmol) HBETI, the mixing ratio of 2PhIm is 0.3842g (2.66mmol) in Example 1-1, and 0.5763g (4.00mmol) in Example 1-2. Be 0.8644g (6.00mmol) in example 1-3, be 1.345g (9.34mmol) in example 1-4, be 2.305g (16.0mmol) in example 1-5, in example 1-6 It was 5.187g (36.0mmol). The mixing process was carried out in a glove box under an argon (Ar) atmosphere, a moisture concentration of 10 ppm or less, and a temperature of 150° C. (equal to or higher than the melting point of 2 PhIm (136° C.)) for...
Embodiment 2-1 and 2-2
[0135] A mixture was formed as in Examples 1-2 and 1-4, except that 4-phenylimidazole (hereinafter referred to as "4PhIm") was used as the first compound. Thereafter, as in Examples 1-2 and 1-4, the melting point and glass transition temperature were determined by DSC measurement, and the water solubility and ion conductivity were determined by AC impedance measurement. The results are shown in Table 2 and Figure 4. Also shown in Figure 4 are the results of Examples 1-2 and 1-4.
[0136] First
[0137] As shown in Table 2, the melting point of each mixture was found to drop to a level below the melting point of 4PhIm (129°C). Further, these mixtures were found to be insoluble in water. And as shown in FIG. 4 , it was found that at a temperature of 110° C. or higher, the same ion conductivity as that of Examples 1-2 and 1-4 was exhibited. That is, it was found that, as in Examples 1-1 to 1-6, even when other imidazoles were used, the mixture was insoluble in wat...
Embodiment 3-1-1、3-1-2、3-2-1 and 3-2-2
[0139] In addition to using 2-isopropylimidazole shown in Chemical Formula 17 (hereinafter referred to as "2-i-PrIm") or 2-n-propylimidazole shown in Chemical Formula 19 (hereinafter referred to as "2-n-PrIm") ") As the first compound, a mixture was formed as in Examples 1-2 and 1-4. Thereafter, as in the case of Examples 1-2 and 1-4, melting point and glass transition temperature were determined by DSC measurement, and water solubility and ion conductivity were determined by AC impedance measurement. The results are shown in Table 3 and Figure 5. In Fig. 5, the ionic conductivities of the mixtures in Examples 3-1-2 and 3-2-2, which are typical examples at room temperature, are shown together with the ionic conductivities of Examples 1-4 As a liquid, the molar ratio of 2-i-PrIm or 2-n-PrIm to HBETI is 7:3.
[0140] First
compound
second
compound
mix
Proportion
melting point
Example ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com