Ahylysantinfarctase 36KD single-stranded haemocoagulase and its preparing method
A technology for hemagglutinin and scutellaria, which is applied in the field of separation and purification to obtain hemagglutinin and its preparation, and achieves the effects of stable preparation process, shortened coagulation time and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1, 20060418 batches of Agkistrodon acutus venom 3.6KD hemagglutinase production example
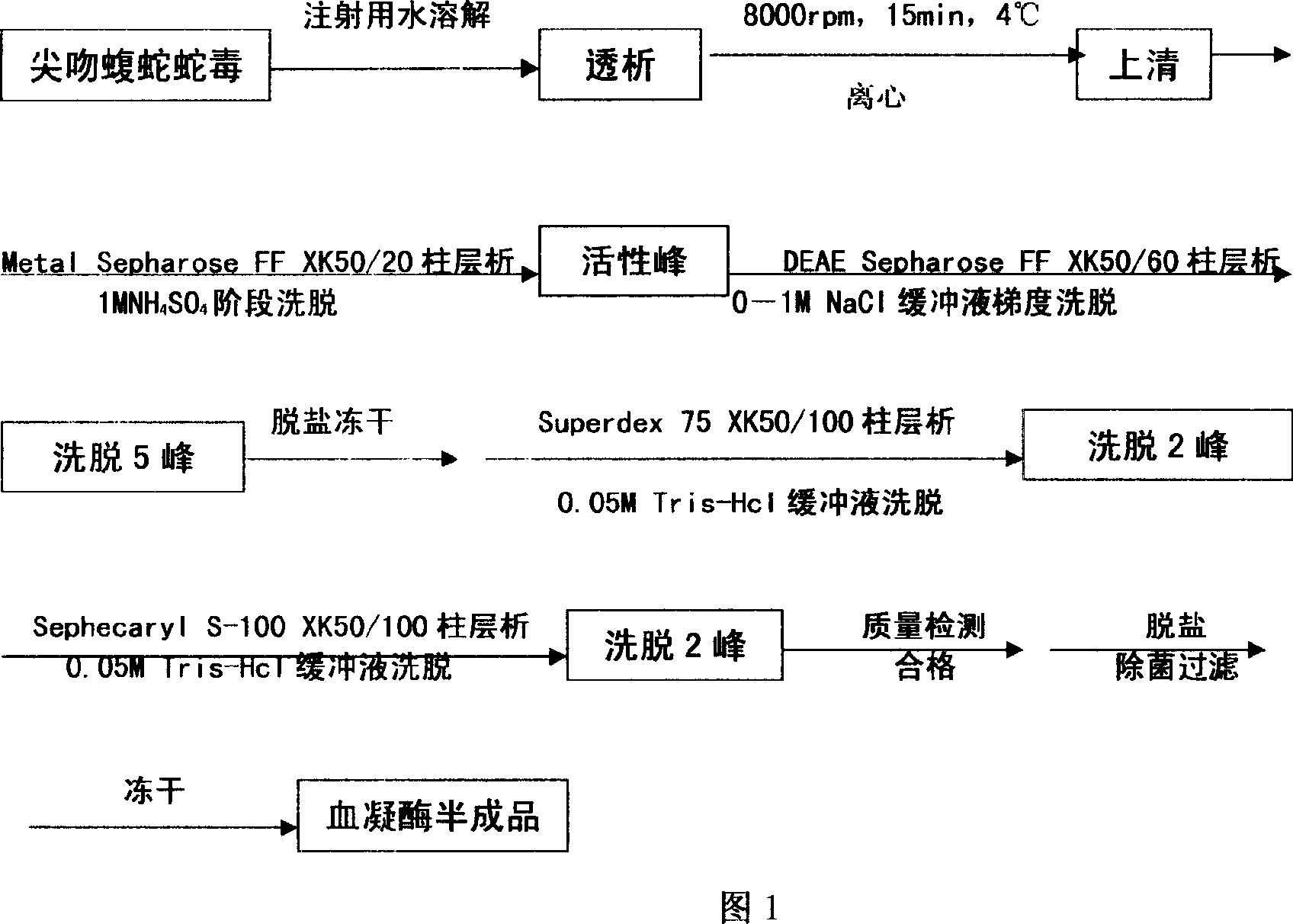
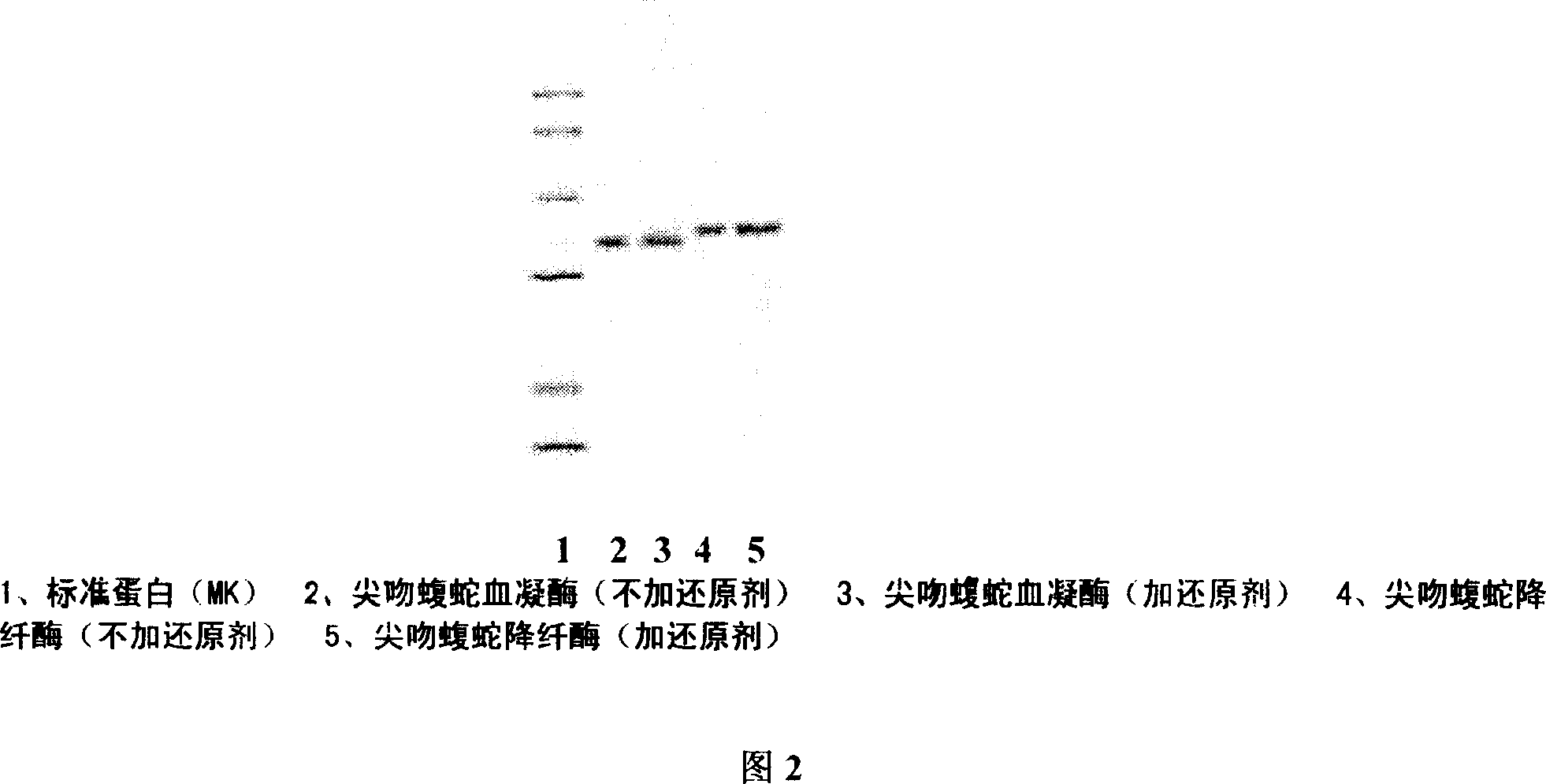
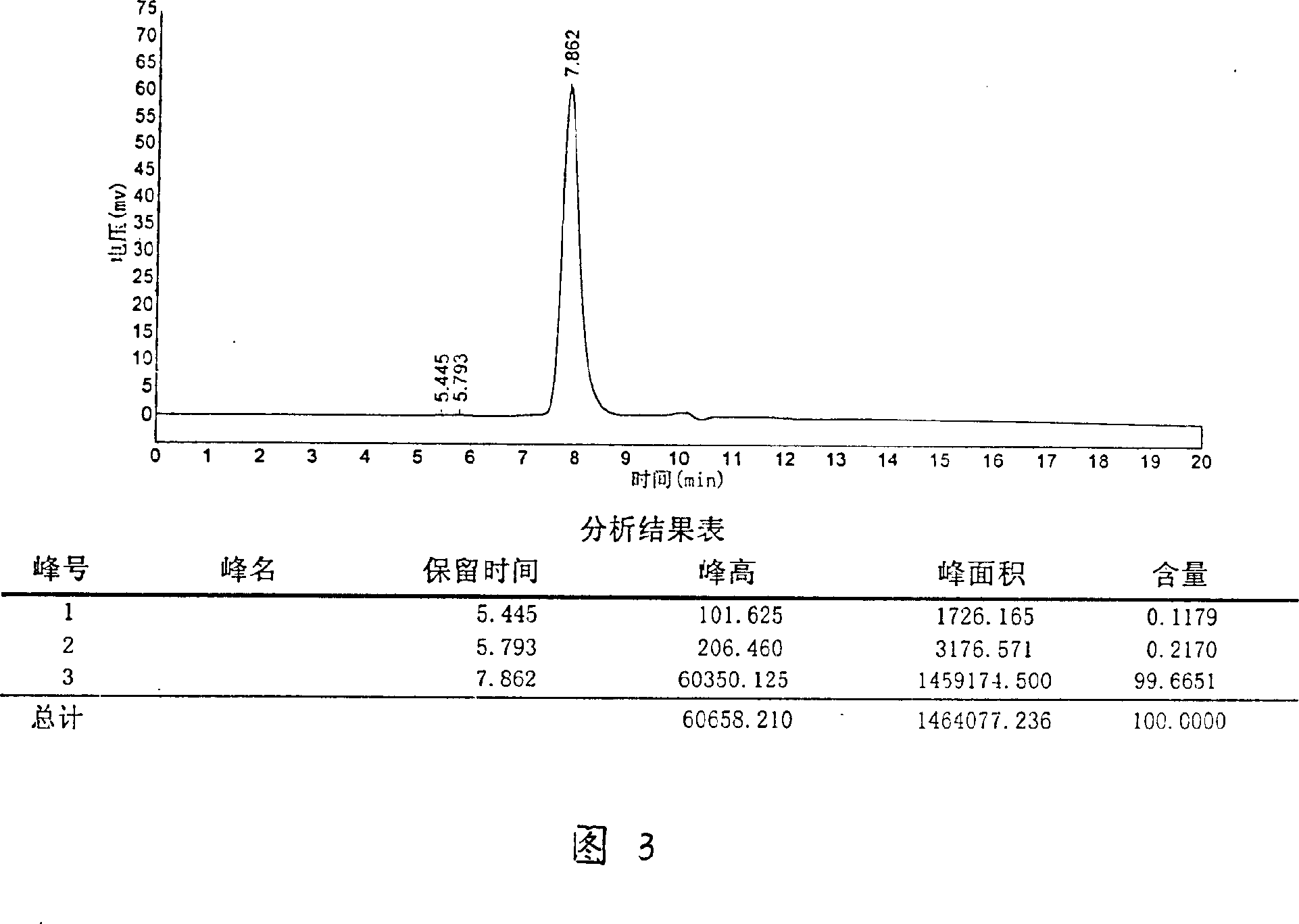
[0032] Weigh 10g of Agkistrodon venom freeze-dried product, add 50ml of sterile water for injection, dialyze with deionized water at 4°C overnight, centrifuge at 8000rpm, 15min, 4°C, and adjust the conductivity of the supernatant to 3.5 with 1M Tris-HCl (PH8.0) After ms / cm, put on the affinity chromatography column Metal Sepharose F.F column which was pre-equilibrated with 0.05M Tris-HCl (PH8.0), 1M NH 4 Cl was eluted in stages, and the breakthrough peak was collected; the DEAE-Sepharose column on the breakthrough peak was equilibrated, and after zero washing with 0.05M Tris-HCl (PH8.0) for 1 column bed volume, wash with 0.05M Tris-HCl+1.0 M NaCl (PH8.0) was used for gradient elution, and the eluted 5 peaks (that is, the crude fraction of hemocoagulase) were collected under the detection of ultraviolet 280nm, and the active peak was desalted and freeze-dried. The lyophi...
Embodiment 2
[0033] Embodiment two, 20060428 batches of Agkistrodon acutus venom 3.6KD hemagglutinase production example
[0034] Weigh 10g of Agkistrodon venom freeze-dried product, add 50ml of sterile water for injection, dialyze with deionized water at 4°C overnight, centrifuge at 8000rpm, 15min, 4°C, and adjust the conductivity of the supernatant to 3.5 with 1M Tris-HCl (PH8.0) After ms / cm, put on the affinity chromatography column Metal Sepharose F.F column which was pre-equilibrated with 0.05M Tris-HCl (PH8.0), 1M NH 4 Cl was eluted in stages, and the breakthrough peak was collected; the DEAE-Sepharose column on the breakthrough peak was equilibrated, and after zero washing with 0.05M Tris-HCl (PH8.0) for 1 column bed volume, wash with 0.05M Tris-HCl+1.0 M NaCl (PH8.0) was used for gradient elution, and the eluted 5 peaks (that is, the crude fraction of hemocoagulase) were collected under the detection of ultraviolet 280nm, and the active peak was desalted and freeze-dried. The lyop...
Embodiment 3
[0035]Embodiment three, 20060523 batches of Agkistrodon acutus venom 3.6KD hemagglutinase production example
[0036] Weigh 10g head of Agkistrodon venom freeze-dried product, add 50ml sterile water for injection, dialyze with deionized water overnight at 4°C, centrifuge at 8000rpm, 15min, 4°C, and use 1M Tris-HCl (PH8.0) to adjust the conductivity of the supernatant to After 3.3ms / cm, put on the Metal sepharose F.F XK50 / 30 column (product of GE Healthcare) equilibrated with 0.05MTris-HCl (PH8.0) in advance, 1M NH 4 Cl was eluted in stages, and the breakthrough peak was collected; the DEAE-sepharose column on the breakthrough peak was equilibrated, and after zero washing with 0.05M Tris-HCl (PH8.0) for 1 column bed volume, wash with 0.05M Tris-HCl+1.0 M NaCl (PH8.0) was used for gradient elution, and the eluted 5 peaks (that is, the crude fraction of hemocoagulase) were collected under the detection of ultraviolet 280nm, and the active peak was desalted and freeze-dried. Diss...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com