Clean method for producing high-purity laminine
A technology of laminin and its production method, which is applied in the field of clean production of high-purity laminin, and can solve problems such as affecting product purity, increasing by-products, and overloading of single-stage ion exchange columns.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
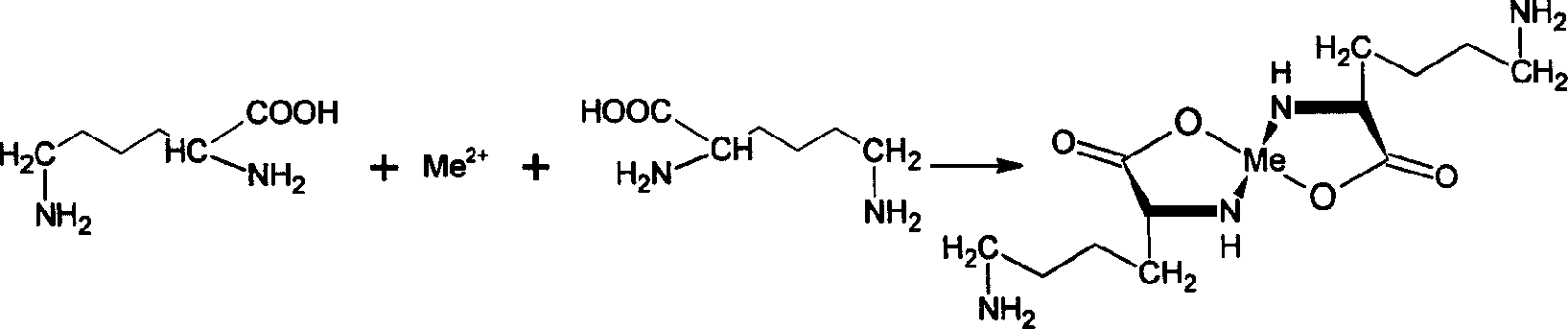
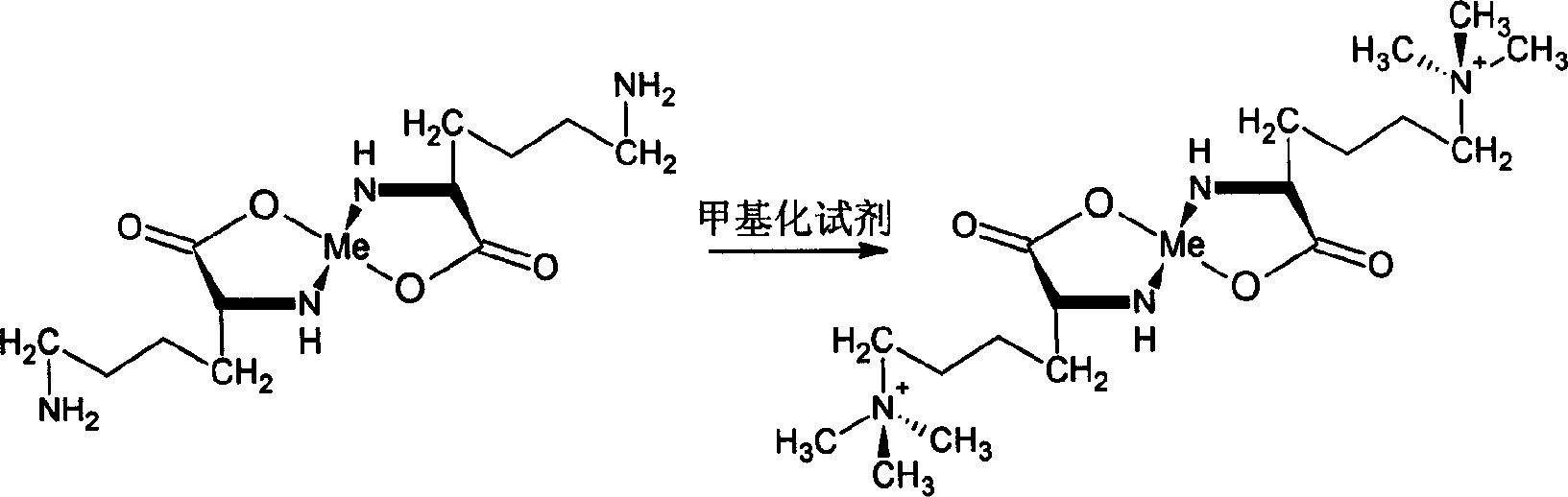
[0026] In a 100L reaction kettle, first add 70 kg of distilled water, add 18 kg of L-lysine hydrochloride into the reaction kettle under stirring and dissolve completely, then add ammonia water to adjust the pH value to 7, then slowly add 25 kg of zinc sulfate , React at a constant temperature of 60°C for 1, 1.5 or 2 hours to allow lysine to react completely with zinc ions; then react with 28 kg of dimethyl carbonate to obtain a mixed solution of laminin.
[0027] Pass the laminin complex mixed solution through a strongly acidic cation resin exchange column to adsorb the laminin complex, and wash to remove impurities such as lysine, monomethylated lysine, and dimethylated lysine.
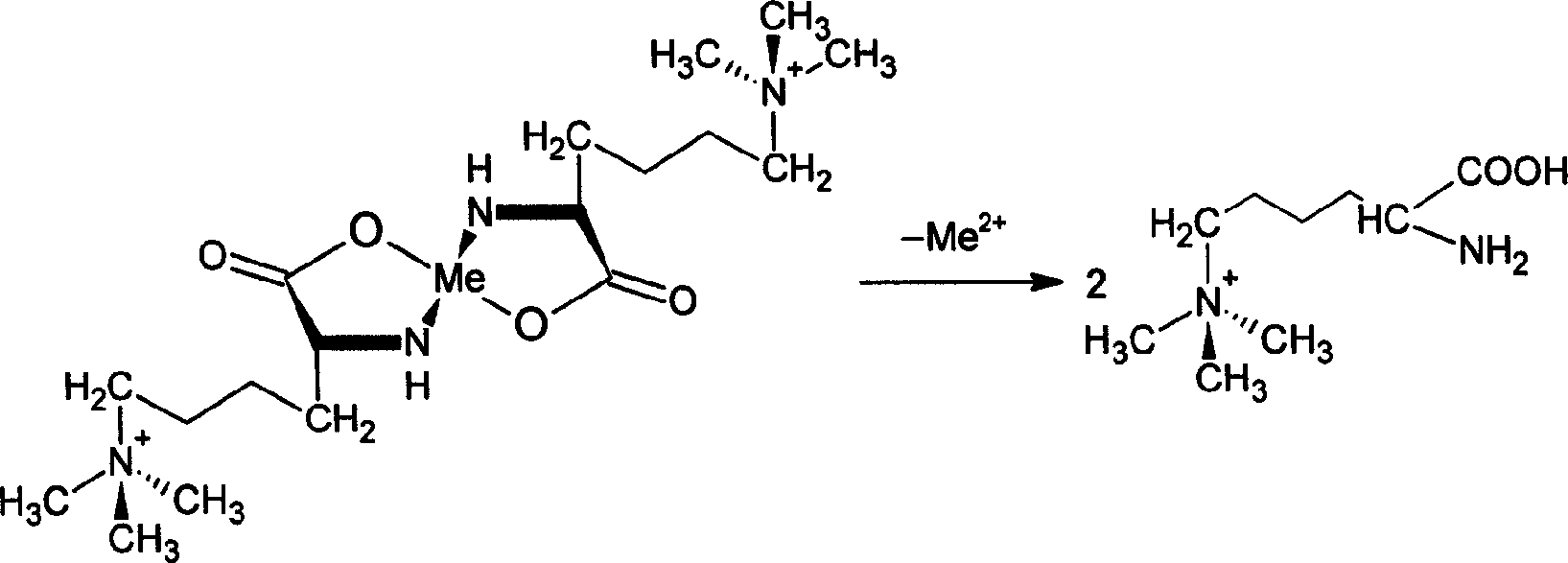
[0028] Add 14 kg of ethylenediaminetetraacetic acid disodium salt to the laminamic acid complex solution obtained by desorption to produce a metal ion demasking reaction to obtain a laminamic acid mixed solution, and pass the mixed solution through the second strongly acidic cation resin exchange col...
Embodiment 2
[0030] In a 500L reactor, first add 360 kg of distilled water, add ammonia water to adjust the pH value to 9, add 95 kg of L-lysine hydrochloride to the reactor to dissolve under stirring, add ammonia water to adjust the pH value to 9, 10 or 11. Add 105 kg of zinc sulfate and react at a constant temperature of 60°C for 1, 1.5 or 2 hours to allow lysine to react completely with zinc ions; then react with 121 kg of dimethyl carbonate to obtain a mixed solution of laminin.
[0031] Pass the laminin complex mixed solution through a strongly acidic cation resin exchange column to adsorb the laminin complex, and wash to remove impurities such as lysine, monomethylated lysine, and dimethylated lysine.
[0032] Add 72 kg of ethylenediaminetetraacetic acid disodium salt to the laminamic acid complex solution obtained by desorption to produce a metal ion demasking reaction to obtain a laminamic mixed solution, and pass the mixed solution through the second strongly acidic cation resin ex...
Embodiment 3
[0034] In a 500L reactor, first add 360 kg of distilled water, add ammonia water to adjust the pH value to 11, add 95 kg of L-lysine hydrochloride into the reactor to dissolve under stirring, add ammonia water to adjust the pH value to 9, 10 or 11. Add 105 kg of zinc sulfate and react at a constant temperature of 60°C for 1, 1.5 or 2 hours to allow lysine to react completely with zinc ions; then react with 121 kg of dimethyl carbonate to obtain a mixed solution of laminin.
[0035] Pass the laminin complex mixed solution through a strongly acidic cation resin exchange column to adsorb the laminin complex, and wash to remove impurities such as lysine, monomethylated lysine, and dimethylated lysine.
[0036] Add 72 kg of ethylenediaminetetraacetic acid disodium salt to the laminamic acid complex solution obtained by desorption to produce a metal ion demasking reaction to obtain a laminamic mixed solution, and pass the mixed solution through the second strongly acidic cation resin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com