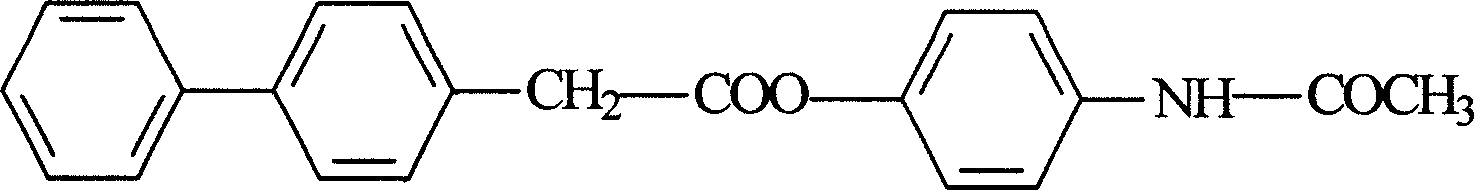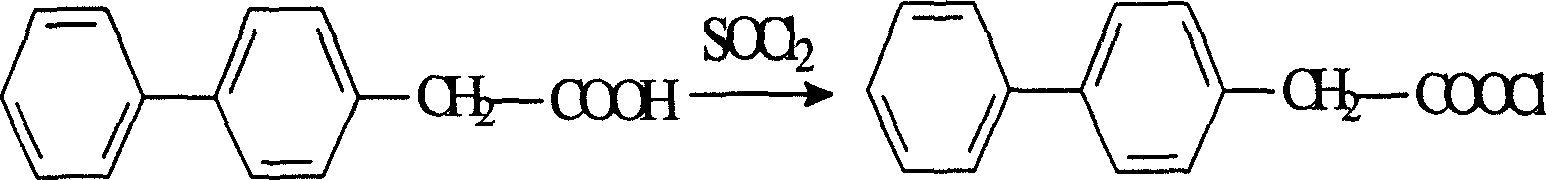Felbinac acetaminopher ester and its preparation method
A technology of acetaminophen ester and felbinac, which is applied in the field of acetaminophen felbinac compound, can solve the problems affecting the popularization and use of drugs, low bioavailability, etc., and achieves simple and feasible synthesis process, good product purity, good analgesic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: Preparation of paracetamol felbinac
[0028] Add 21.2g of felbinac and 120ml of DMF into a 500ml three-necked flask, add 11.9g of thionyl chloride dropwise under stirring, stir and reflux for 2 hours after the drop-in is complete, recover excess thionyl chloride under reduced pressure after the reaction is completed, and collect bp : 123-125°C fraction, 18.7g, yield 78%.
[0029] In a 250ml three-neck flask, add 9g of paracetamol, 2ml of pyridine, 100ml of acetone, add 50ml of ethyl acetate and 18.7g of biphenylacetamide under stirring, and stir for 3 hours at high temperature, fully cool in an ice bath, and precipitate by suction filtration The solid was washed with a small amount of cold absolute ethanol, and dried to obtain 23.5 g of white crude crystals. Yield 63%.
[0030] Put the above crude crystals in a 250ml round-bottomed flask, add 150ml of absolute ethanol and 0.5g of activated carbon, heat to reflux for 2min, decarburize by suction filtratio...
Embodiment 2
[0036] Embodiment 2: Preparation of paracetamol felbinac
[0037] Operation is by embodiment 1, feed intake is:
[0038] Felbinac: 21.2g
[0039] Thionyl chloride: 119g
[0040] Acetaminophen: 45g
[0041] 17.9 g of paracetamol felbinac was obtained, m.p.: 138.6-139.1° C., yield: 84.4%.
[0042] Embodiment 2: Preparation of paracetamol felbinac
[0043] Operation is by embodiment 1, feed intake is:
[0044] Felbinac: 21.2g
[0045] Thionyl chloride: 40g
[0046] Acetaminophen: 18g
[0047] 19.8 g of paracetamol felbinac was obtained, m.p.: 138.5-139.3°C, yield: 93.4%.
Embodiment 3
[0048] Embodiment 3: the preparation of paracetamol felbinac
[0049] In a 250ml three-neck flask, add 21.2g of felbinac and 150ml of acetone and drop in 40ml of thionyl chloride under stirring. After the drop is completed, reflux for 2 hours, recover excess thionyl chloride under reduced pressure, and collect bp: 123- The fraction at 125°C / 133Pa yielded 19.3g, with a yield of 80%.
[0050] In a 250ml three-necked flask, add 9.6g of acetaminophen, 2ml of pyridine, 120ml of acetone and 60ml of ethyl acetate, and drop in 19.3g of biphenylacetyl chloride under stirring, stir at high temperature for 3 hours after the dropping, and fully cool in the refrigerator After that, the solid was precipitated by suction filtration, washed and dried with a small amount of absolute ethanol to obtain 259 g of white crude crystals. Yield: 64.2%.
[0051]Put the above crude crystals in a 250ml round bottom flask, add 160ml of absolute ethanol and 0.5g of activated carbon, heat and reflux for 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com