Construction and use of Q-type human paraoxonase 1 gene expression vector
A paraoxonase and gene technology, applied in the field of biotechnology and pharmaceuticals, can solve the problems of multiple medications, short action time, slow onset, etc., and achieve significant liver protection effect, fast onset, and less toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
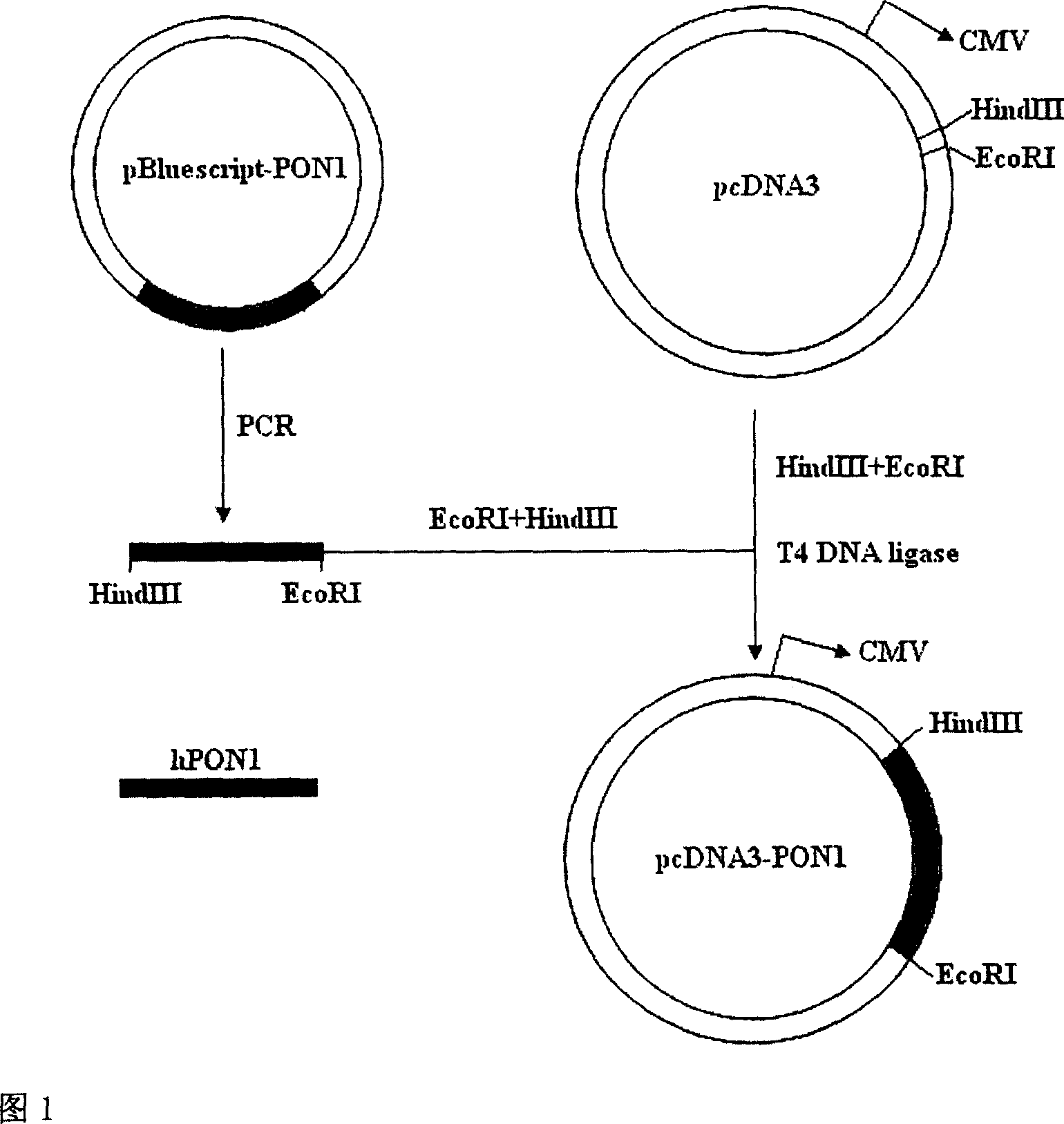
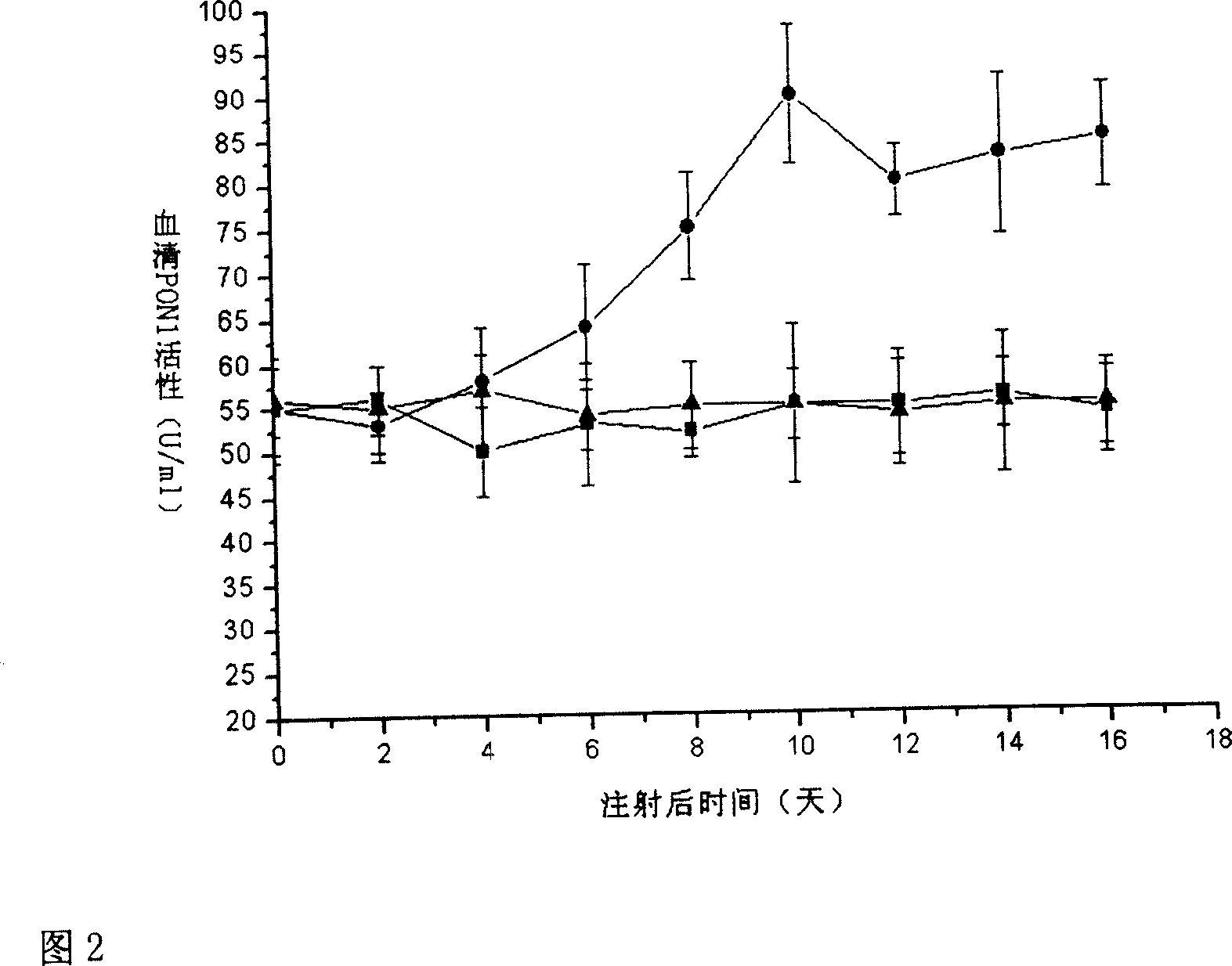
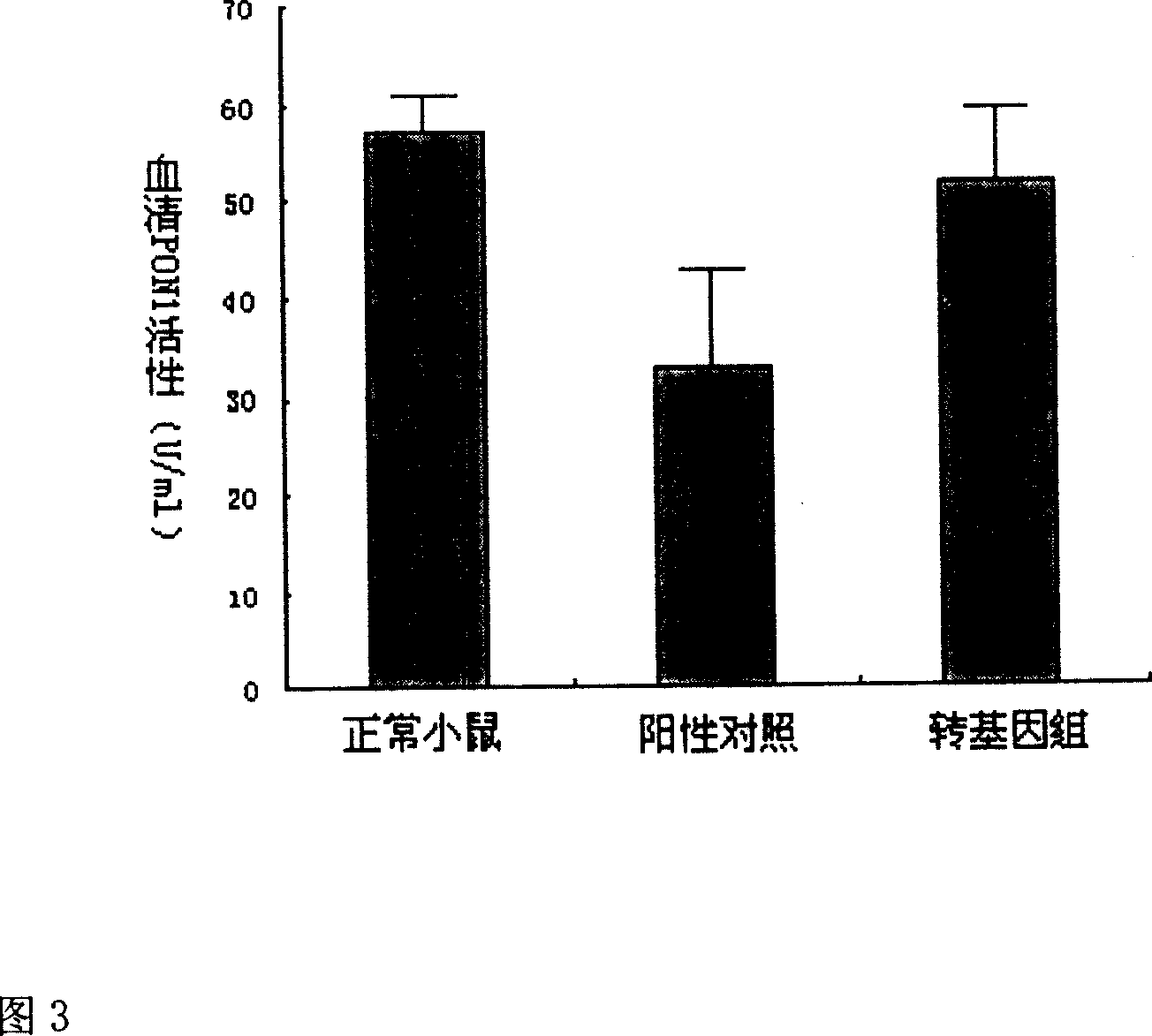
[0028] 1. The pBluescript-hPON1Q plasmid was donated by Professor C.E. Furlong. Using pBluescript-hPON1Q as a template, use primers 1 and 2 to amplify the full-length gene of hPON1Q with a total of 1065 bp by PCR method, and insert it into the multiple cloning site of pcDNA3.0 plasmid after double digestion with EcoR I and HindIII. The hPON1Q gene is under the control of the CMV promoter. Sequencing proved that the result was completely consistent with the designed sequence (see Figure 1 for the process flow). Transform pcDNA3.0-hPON1Q with CaCl 2The competent TOP10 Escherichia coli was prepared by the method, and the Escherichia coli was amplified according to the operation procedure of the third edition of "Molecular Cloning" to extract a large number of plasmids. The purity and yield of the extracted plasmids were determined by UV spectrophotometry, and the plasmid concentration was adjusted to 1 μg / μl with PBS. All biochemical reagents were purchased from Sigma, and enz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com