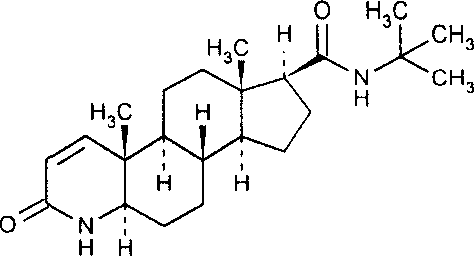
Sustained release microsphere of finasteride and its analogue, preparation process and use thereof
A technology of sustained-release microspheres and finasteride, which is applied to medical preparations containing active ingredients, drug combinations, and pharmaceutical formulas, and can solve problems such as compliance and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
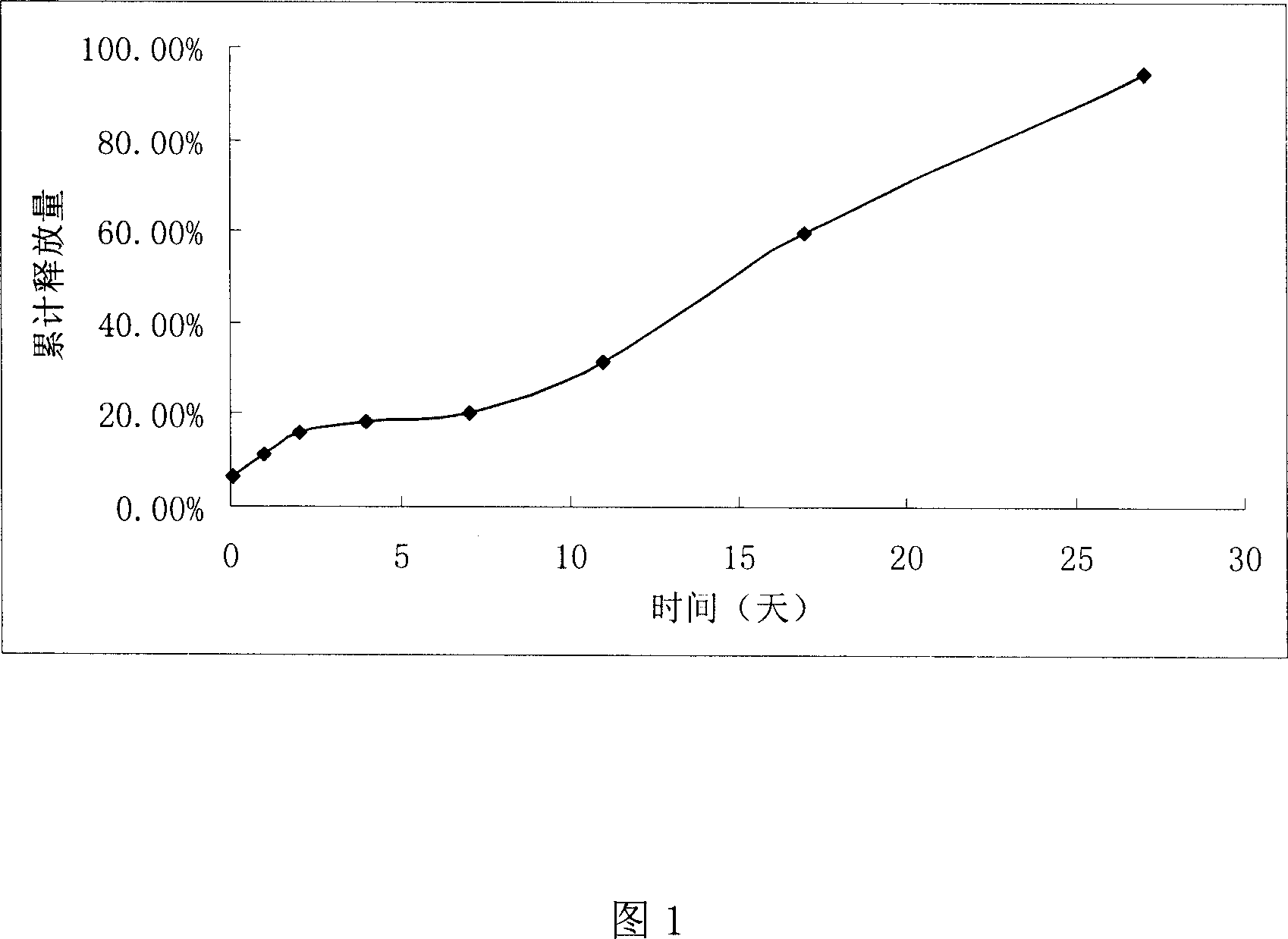
[0028] Dissolve 100mg of polylactide in 0.6ml of dichloromethane, add 100mg of finasteride, stir and dissolve to form a clear solution. Add 0.5% PVA (05-88) aqueous solution into a flat-bottomed beaker equipped with a stirring paddle, keep the temperature (25°C), then drop the above polymer solution into it under stirring, stir at 2000rpm to form an emulsion, then add a large amount of distilled water, Maintain 200rpm to gradually evaporate the organic solvent in the o / w emulsion to dryness. Sampling and microscopic inspection were carried out continuously during the whole process to observe the multi-stage microsphere formation process.
[0029] The above solution is centrifuged, the supernatant is poured off, the microspheres are collected under reduced pressure on a filter membrane, washed with a small amount of distilled water several times, and dried under reduced pressure at room temperature.
Embodiment 2
[0031] Dissolve 160mg of poly-DL-lactide-glycolide in ethyl acetate, add 40mg of finasteride, and stir to form a clear solution. Add a 1% PVPk-30 aqueous solution of 10 times the volume of the dispersed phase into a flat-bottomed beaker equipped with a stirring paddle. After constant temperature (25°C), drop the above polymer solution into it under stirring, stir at 2000rpm to form an emulsion, and then add a large amount of distilled water, and kept stirring at 200 rpm for 4 hours, so that the organic solvent in the o / w emulsion was gradually evaporated to dryness. Sampling and microscopic inspection were carried out continuously during the whole process to observe the multi-stage microsphere formation process.
[0032] The above solution is centrifuged, the supernatant is poured off, the microspheres are collected under reduced pressure on a filter membrane, washed with a small amount of distilled water several times, and dried under reduced pressure at room temperature.
Embodiment 3
[0034] Dissolve 800 mg of polylactic-glycolic acid in 2.5 ml of acetonitrile, and add 200 mg of finasteride. Stir to dissolve. As O1 phase; in 30ml liquid paraffin, add 1.5% Span85, mix well as O2 phase. At 3000rpm, drop O1 into O2 to make O1 / O2 emulsion. Control the temperature of the system, keep the constant temperature at 30°C, and gradually evaporate the organic solvent in the dispersed phase to dryness. The suspension is centrifuged, and the supernatant is removed. The microspheres are washed with a small amount of hexane several times, and dried under reduced pressure at room temperature. .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com