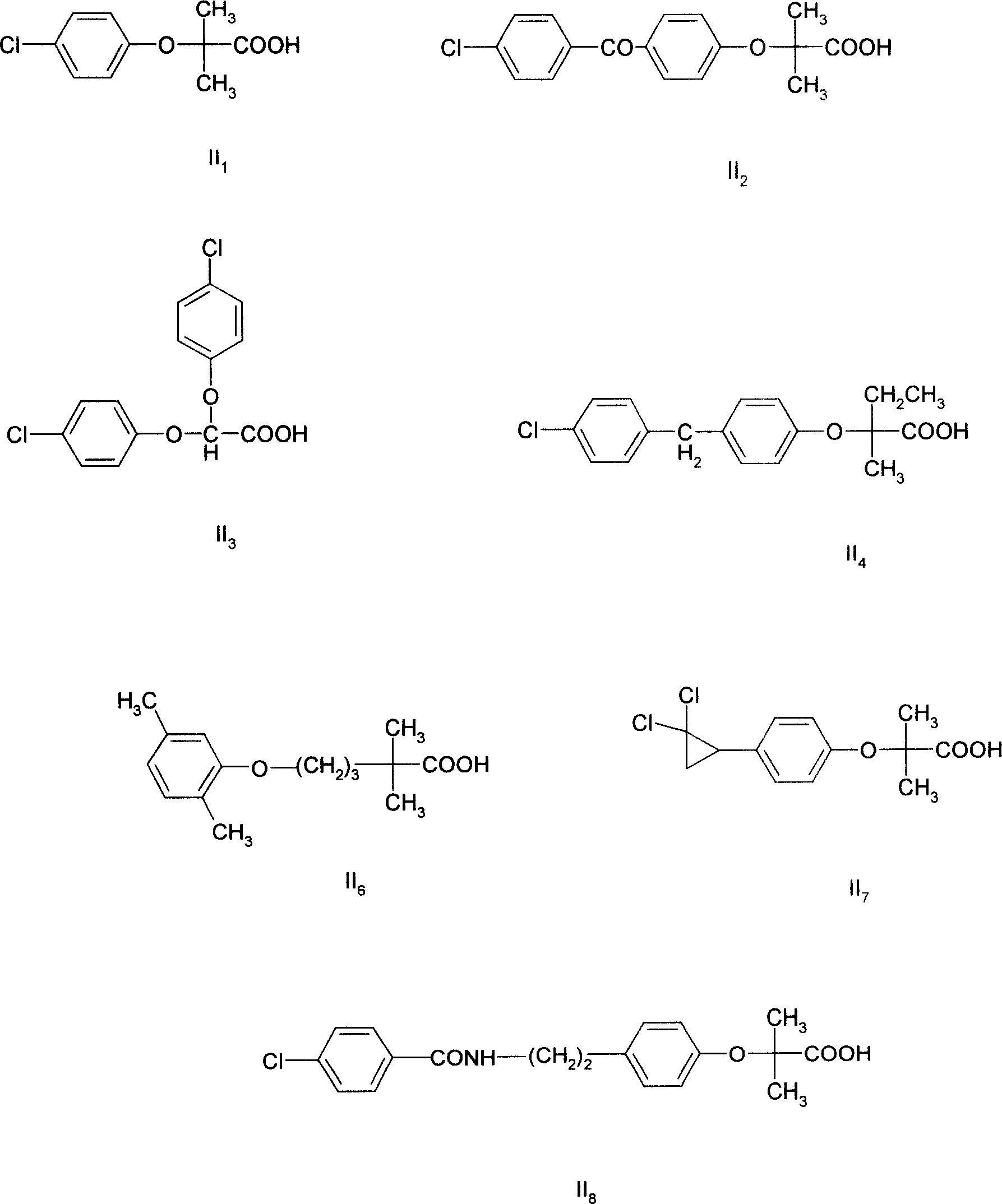New phenoxy eicosanoic acid derivative and its medical use
A technology of phenoxyalkanoic acid and derivatives, which is applied in the field of new phenoxyalkanoic acid derivatives and their medical applications, can solve problems such as elevated transaminases, increase high-density lipoprotein, reduce triglyceride Lipid and low-density lipoprotein levels, hepatoprotective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
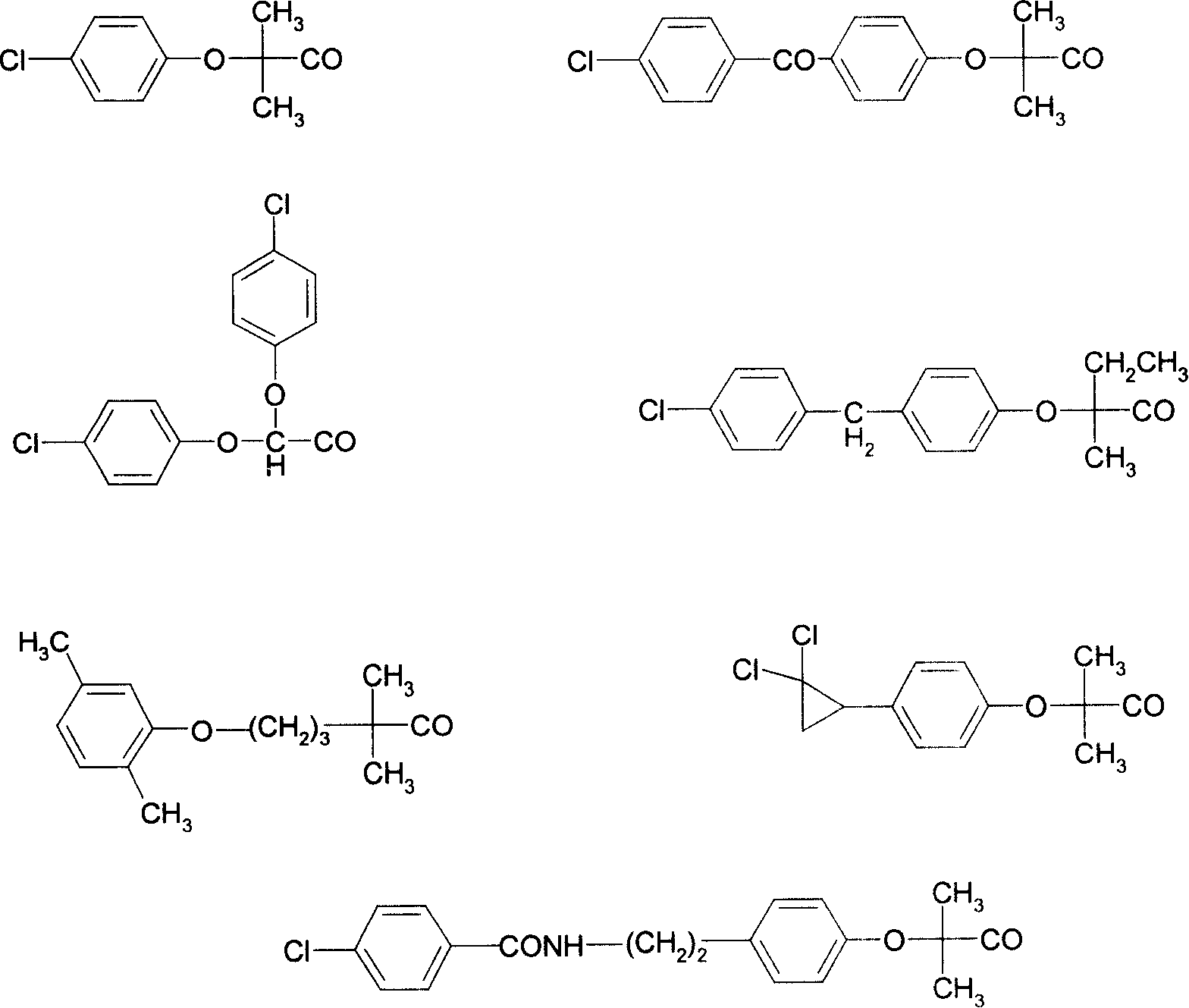
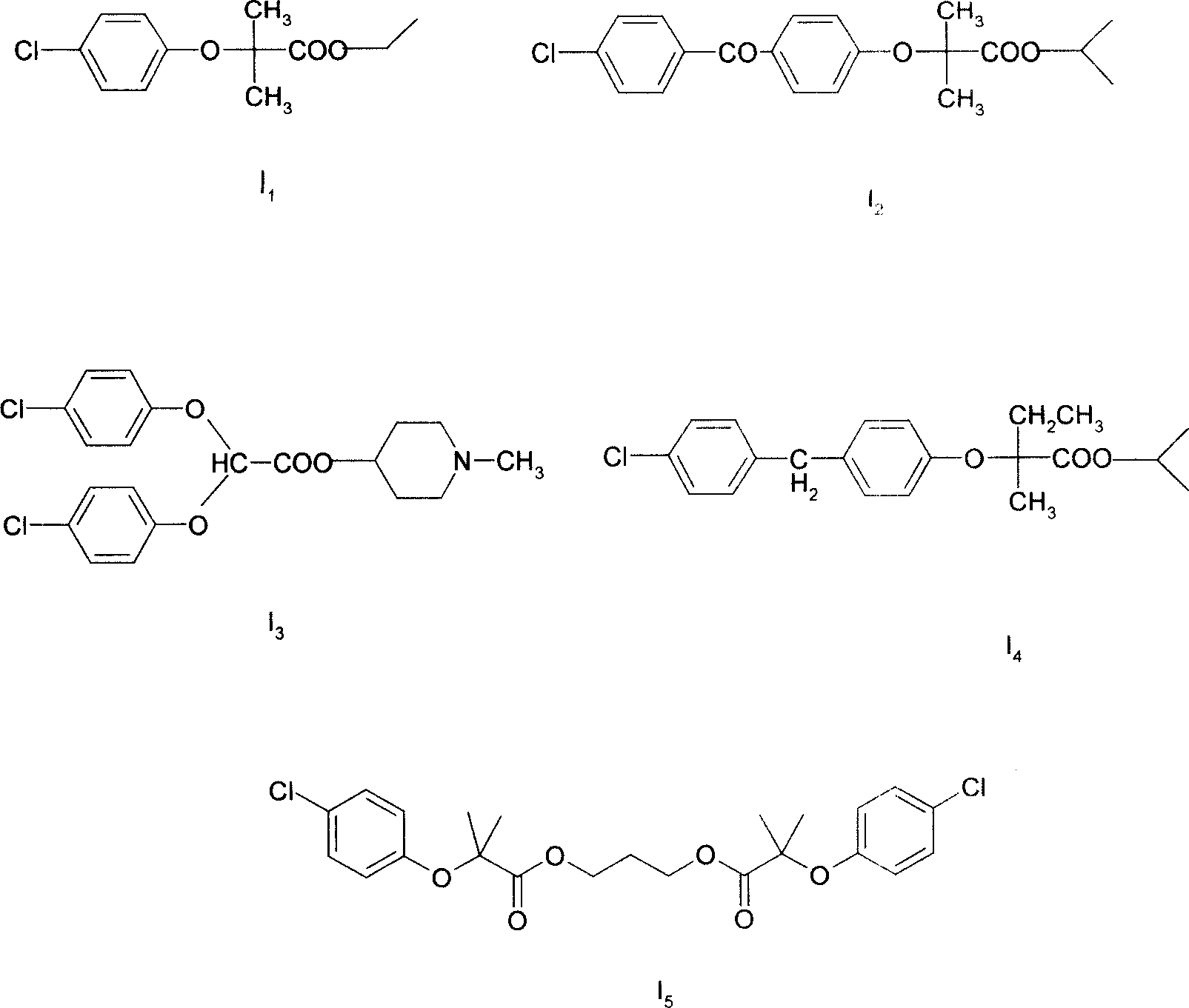
Image
Examples
Embodiment 1
[0016] Example 1 2-Methyl-2-[4-(p-chloro-benzoyl)-phenoxy]-propionyl-(2-nitrooxy-ethyl)-ester (III 1 ) preparation
[0017]
[0018] 1.1 Preparation of 2-methyl-2-[4-(p-chloro-benzoyl)-phenoxy]-sodium propionate
[0019] 36 g (0.1 mol) of fenofibrate (purchased from Sigma) was dissolved in 600 ml of ethanol, and 100 ml of 1M sodium hydroxide solution was added dropwise with stirring. After dripping, stir the reaction at room temperature for 1 hour, evaporate the solvent under reduced pressure, add diethyl ether to wash, and filter to obtain 2-methyl-2-[4-(p-chloro-benzoyl)-phenoxy]-propionic acid Sodium 34 grams.
[0020] 1.2 2-Methyl-2-[4-(p-chloro-benzoyl)-phenoxy]-propionyl-(2-nitrooxy-ethyl)-ester (III 1 ) preparation
[0021] 3.4 g (10 mmol) of 2-methyl-2-[4-(p-chloro-benzoyl)-phenoxy]-sodium propionate was suspended in 50 ml of DMF, and 3.0 g (16 mmol) of 1 , 2-dibromoethane, reacted at room temperature for 22 hours, filtered, the filtrate was evaporated to dryne...
Embodiment 2
[0022] Example 2 2-Methyl-2-[4-(p-chloro-benzoyl)-phenoxy]-propionyl-(3-nitrooxy-propyl)-ester (III 2 ) preparation
[0023]
[0024] According to the method of 1.2, replace 1,2-dibromoethane with 1,3-dibromopropane to obtain III 2 , yield 70%; elemental analysis C 20 h 20 ClNO 7 Calculated (%): C 56.95, H 4.78, N 3.32, Cl 8.40; Found (%): C 56.72, H 4.75, N 3.06, Cl 8.19.
Embodiment 3
[0025] Example 3 2-Methyl-2-[4-(p-chloro-benzoyl)-phenoxy]-propionyl-(4-nitrooxy-butyl)-ester (III 3 ) preparation
[0026]
[0027] According to the method of 1.2, replace 1,2-dibromoethane with 1,4-dibromobutane to obtain III 3 , yield 61%; elemental analysis C 21 h 22 ClNO 7 Calculated (%): C 57.87, H 5.09, N 3.21, Cl 8.13; Found (%): C 57.68, H 4.86, N 3.10, Cl 8.22.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com