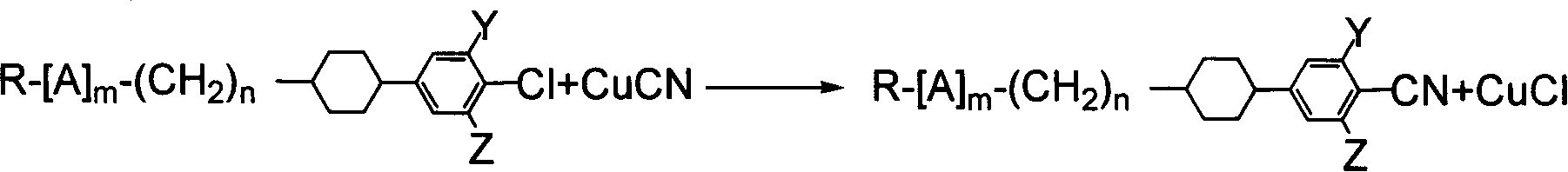
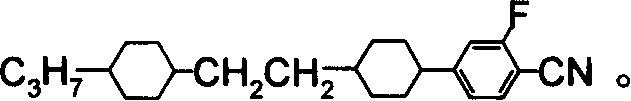
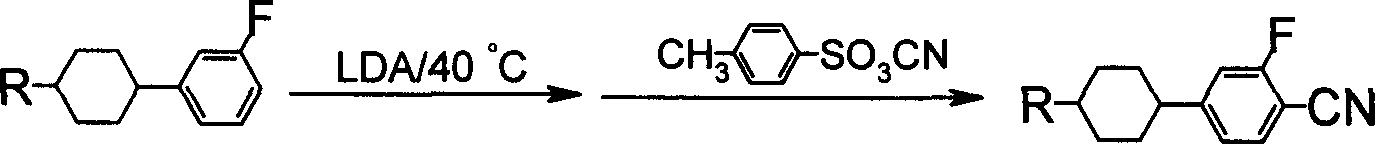
Benzene nitrile monomer liquid crystal preparation method
A technology of benzonitriles and monomers, which is applied in the field of monomer liquid crystal preparation, can solve the problems of environmental pollution damage, high cost of raw materials, waste acid discharge, etc., and achieve the effects of being environmentally friendly, reducing reaction steps, and reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1.1: Raw material 4-(trans-4'-propylcyclohexyl)-2,6-difluorochlorobenzene [structural formula: preparation of
[0028] Add 12.2g (0.5mol) of magnesium chips into a 1L three-necked flask, 113.7g (0.5mol) of 2,6-difluoro-4-bromochlorobenzene is miscible in 300ml of tetrahydrofuran, drop a little into the three-necked flask, and heat up to 70°C, the reaction was initiated, and the remaining 2,6-difluoro-4-bromochlorobenzene solution was added dropwise. After the addition was completed, the mixture was kept at reflux for 1 hour. Dissolve 70.0g (0.5mol) of p-propylcyclohexanone in 100ml of tetrahydrofuran, drop it into the Grignard reagent obtained from the above reaction, after the addition is complete, keep it warm for 1 hour, then add dilute hydrochloric acid for hydrolysis, and extract it three times with 100ml of toluene , combine the organic phases, wash with water until neutral, add 8.6g (0.05mol) of p-toluenesulfonic acid into the above-mentioned toluene solution, ...
Embodiment 2
[0036] 2.1: Raw material 4-〖trans-4′-[2″-(trans-4-propylcyclohexyl)ethylene]cyclohexyl〗2-fluorochlorobenzene, [structural formula: ] Preparation:
[0037] With 123g (0.5mol) of and 104.7g (0.5mol) Grignard reagent reaction, prepared Then use 8.6g (0.05mol) of p-toluenesulfonic acid to dehydrate to obtain Double bond hydrogenation with Raney Ni yielded 170 g of mixed cis and trans GC: 95%, then use 58.7g (0.44mol) of aluminum trichloride, 72g sherwood oil < 5 ℃ condition conversion, then recrystallize three times with the mixed solvent of 1ml toluene and 1mol industrial ethanol / 1g product, obtain 136g
[0038] 4-〖trans-4′-[2″-(trans-4_-propylcyclohexyl) ethylene]cyclohexyl〗2-fluorochlorobenzene, structural formula: GC: 99.5%.
[0039] 2.2: Product 4-〖trans-4′-[2″-(trans-4_-propylcyclohexyl)ethyl]cyclohexyl〗2-fluorobenzonitrile [structural formula: ] preparation
[0040]136g (0.37mol) of 4-[trans-4'-[2"-(trans-4-propylcyclohexyl) ethylene group] cyclohexyl] 2-flu...
Embodiment 3
[0043] 3.1: According to the similar method of Example 2.1, the following compounds can be obtained
[0044] 4-〖trans-4′-[2″-(trans-4_-ethylcyclohexyl) ethylene]cyclohexyl〗2-fluorochlorobenzene, GC: 99.5%, yield: 75%, structural formula:
[0045] 4-〖trans-4′-[2″-(trans-4_-butylcyclohexyl)ethylene]cyclohexyl〗2-fluorochlorobenzene, GC: 99.5%, yield: 74%, structural formula:
[0046] 3.2: According to the similar method of Example 2.2, the following monomeric liquid crystal compounds can be obtained
[0047] 4-〖trans-4′-[2″-(trans-4_-ethylcyclohexyl)ethylene]cyclohexyl〗2-fluorobenzonitrile, mp: 70.6°C, cp: 145.6°C, [structural formula: ].
[0048] 4-〖trans-4′-[2″-(trans-4_-butylcyclohexyl)ethylene]cyclohexyl〗2-fluoro-benzonitrile, mp: 54.9°C, cp: 165.7°C, [structural formula: ].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com