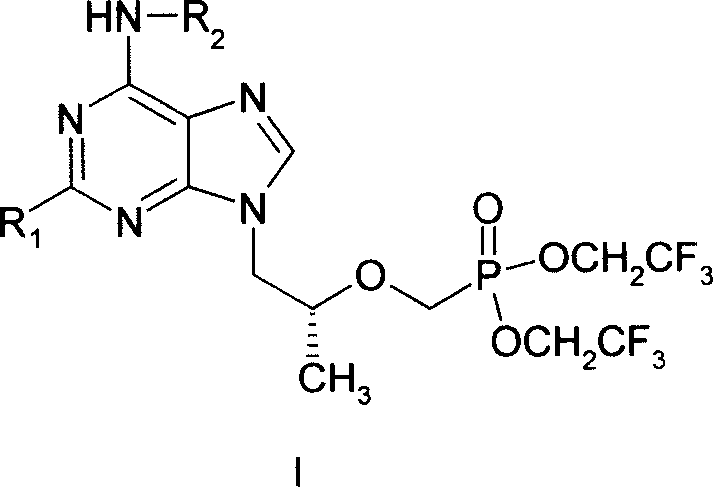
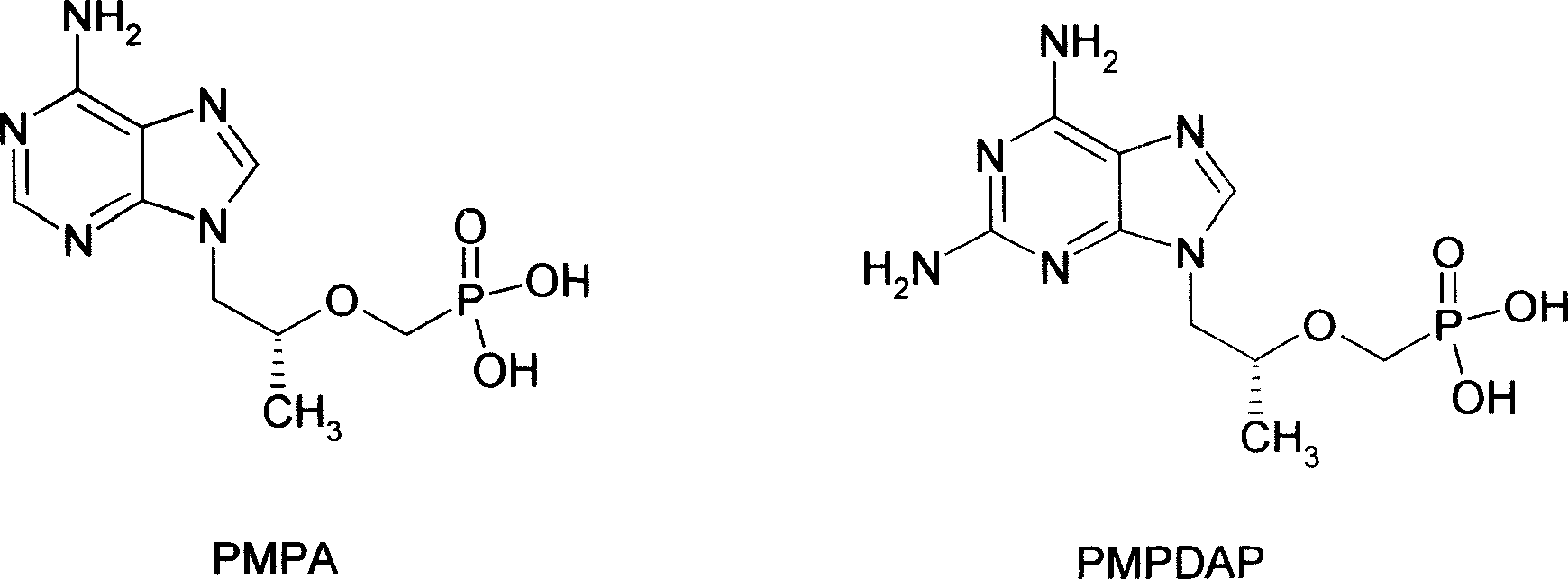
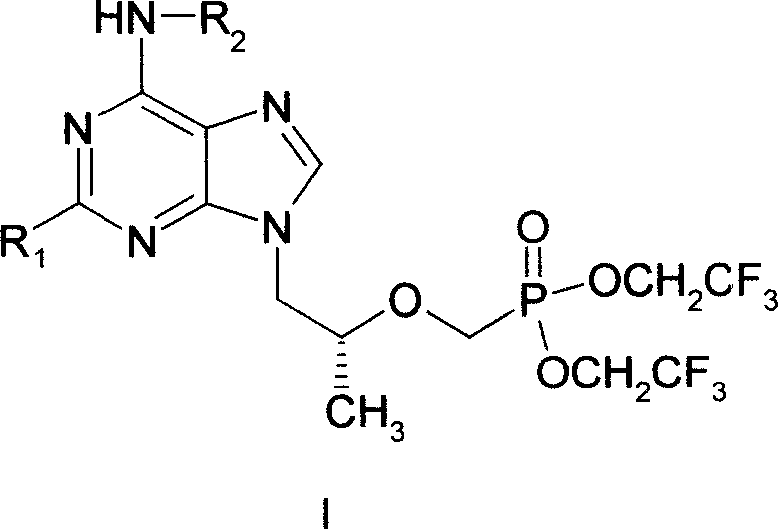
Novel acyclic nucleoside phosphonate and its medical use
A nucleoside phosphonate and pharmaceutical technology, applied in the field of acyclic nucleoside phosphonate and its non-toxic pharmaceutically acceptable salts, can solve the problems of low oral bioavailability and difficulty in reaching effective therapeutic concentrations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1 9-{2-[bis-(trifluoroethyl)-phosphonomethoxy]-propyl}-adenine (I 1 ) preparation
[0015] 1.1 Synthesis of (R)-1-chloro-2-propanol
[0016] Add 208 g (2 mol) of (R)-methyl lactate to 1200 ml of dimethylformamide, cool in an ice bath, add 96 g (2.4 mol) of 60% sodium hydride in batches under stirring, and react with stirring for 1 hour. Then 408 g (2.4 mol) of benzyl bromide was added dropwise, and after the drop was completed, the mixture was stirred under ice bath for 4 hours, and continued to stir at room temperature for 48 hours. DMF was evaporated under reduced pressure, and the residue was separated by silica gel column chromatography, eluting with ethyl acetate:petroleum ether (1:9), and the desired components were collected and evaporated to dryness under reduced pressure to obtain 188 g of an oily liquid.
[0017] Dissolve 186 g (0.98 mol) of the product from the previous step in 1200 ml of anhydrous tetrahydrofuran, place in an ice bath, add 60 g of ...
Embodiment 2
[0026] Example 2 (R)-9-{2-[bis--(2,2,2-trifluoroethyl)-phosphonomethoxy]-propyl}-2,6-diaminopurine (I 2 ) preparation
[0027] According to the method of Example 1.4, replace adenine with 2,6-diaminopurine, and (R)-2-[bis-(2,2,2-trifluoroethyl)phosphonomethoxy]-propyl Chlorine reaction, prepared (R)-9-{2-[bis--(2,2,2-trifluoroethyl)-phosphonomethoxy]-propyl}-2,6-diaminopurine ( I 2 ). Proton NMR spectrum: δ(DMSO-d 6 , ppm): 7.85 (s, 1H); 5.78 (s, 2H, disappeared after heavy water exchange); 5.32 (s, 2H, disappeared after heavy water exchange); 4.69 (m, 4H); 4.58 (m, 1H); 4.21 (d, 2H); 4.06 (d, 2H); 1.23 (d, 3H).
Embodiment 3
[0028] Example 3 (R)-9-{2-[bis-(trifluoroethyl)-phosphonomethoxy]-propyl}-6-methoxycarboxamido-purine (I 3 ) preparation
[0029] 1 g I 1 Dissolve in 30 ml of dichloromethane, add 2.0 ml of anhydrous pyridine, and cool the reaction solution to -40°C. A solution of 0.4 mg of methyl chloroformate in 10 ml of dichloromethane was added dropwise with stirring. Stir for 30 minutes after the addition is complete, and for another 30 minutes at room temperature. The reaction solution was evaporated to dryness, and the residue was separated by silica gel column chromatography, and eluted with chloroform:methanol (96:4) to give I 5 440 mg, H NMR: δ(DMSO-d 6 , ppm): 10.0 (s, 1H, disappear after heavy water exchange); 8.65 (s, 1H); 8.36 (s, 1H); 4.63-4.69 (m, 4H); 4.54 (m, 1H); 4.18 (d, 2H); 4.06(d, 2H); 3.76(s, 3H); 1.24(d, 3H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com