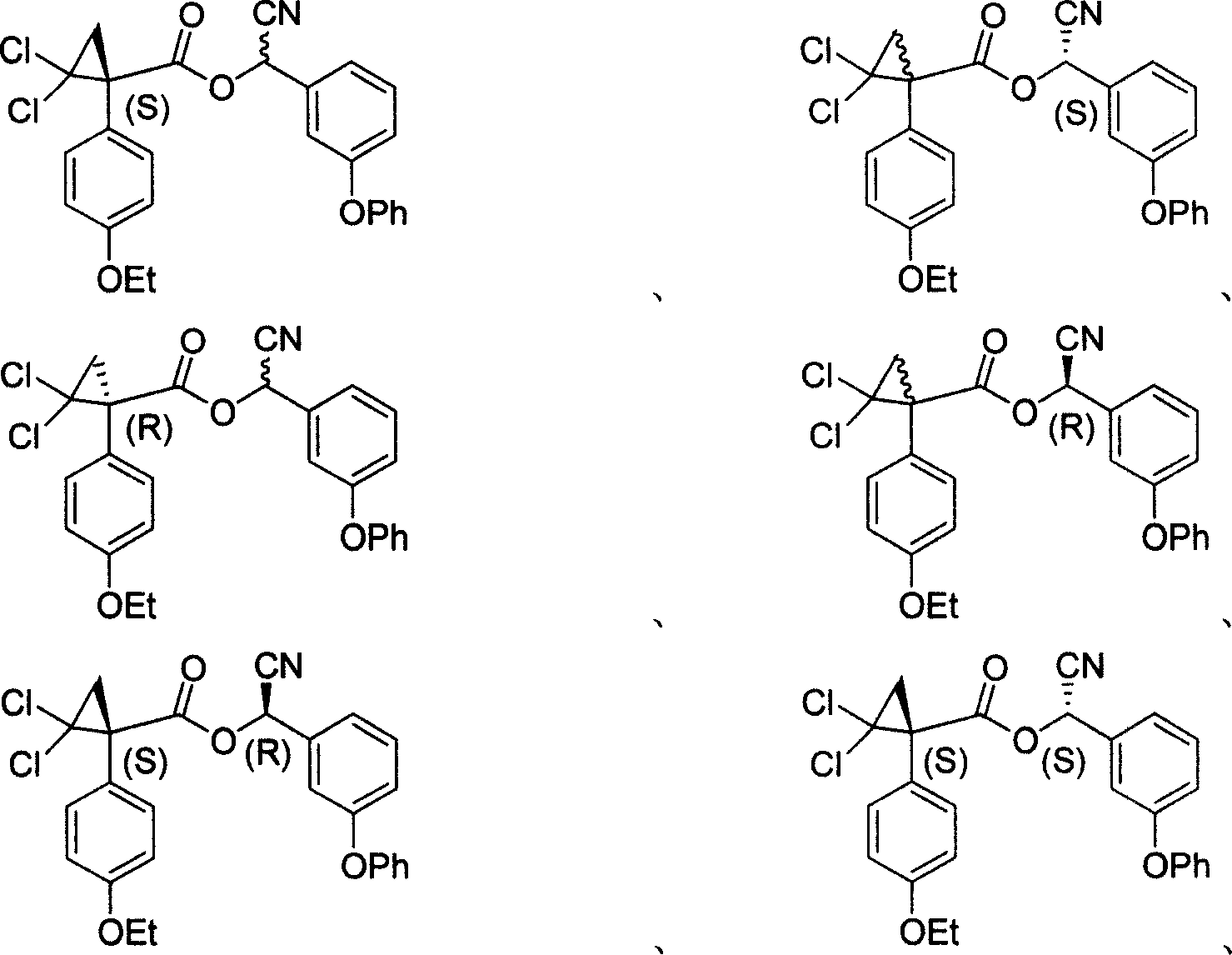
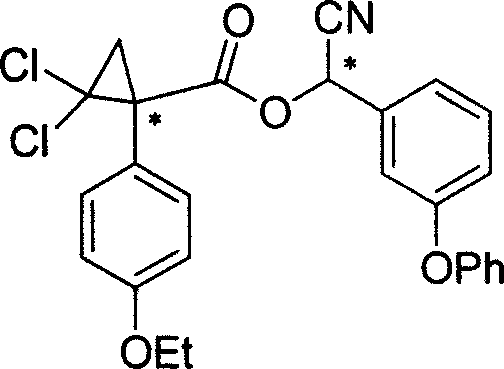
Cycloprothrin optical activity isomer, preparation method and uses
A technology of promethrin and its isomers, which is applied in the field of photoactive isomers, preparation and application of promethrin, can solve the problems of low activity and phytotoxicity, so as to improve drug efficacy and save raw materials , Improve the effect of economic and social benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Preparation of chiral 1-(4-ethoxyphenyl)-2,2-dichlorocyclopropane-1-carboxylic acid
[0037] Under the reaction conditions of 40°C, the racemic 1-(4-ethoxyphenyl)-2,2-dichlorocyclopropane-1-carboxylic acid (3.0g) was mixed with S-(-)-α -Methylbenzylamine (1.5 mL) or R-(+)-α-methylbenzylamine (1.5 mL) was reacted in a solvent of ethyl acetate (25 mL). The reaction continued for about 4 days, and the carboxylic acid amine salt crystals were obtained by filtration. The obtained crystals were crystallized several times in ethyl acetate at room temperature (15-25° C.), and finally needle-shaped crystals were obtained. The crystals were dissolved in ethanol, and 1N dilute hydrochloric acid was added thereto. After a period of time, R-(-)-1-(4-ethoxyphenyl)-2,2-dichlorocyclopropane-1-carboxylic acid [α] D 22.6 =-67.3580 (C.1.0, CHCl 3 , 589nm) or crystals of S-(+)-1-(4-ethoxyphenyl)-2,2-dichlorocyclopropane-1-carboxylic acid [α] D 24.3 =66.5620 (C.1.0, CHCl 3...
Embodiment 2
[0038] Embodiment 2 prepares S-(-)-2-hydroxyl-2-(3-phenoxyphenyl) acetonitrile
[0039] Racemic acetylated 2-hydroxy-2-(3-phenoxyphenyl)acetonitrile (38 mg, 0.14 mmol), butanol (12 mg), and lipase from Pseudomonas sp. (4 mg) were dissolved in isopropyl ether ( 3ml), stirred under the condition of 35°C, followed by dot plate, and after about 8 hours, the lipase was filtered off. After separation by column chromatography, S-(-)-2-hydroxy-2-(3-phenoxyphenyl)acetonitrile was obtained with a yield of 80%. 1 HNMR (CDCl 3 , 300MHz) δ=7.43-7.04(m, 9H), 6.38(s, 1H), 2.16(s, 3H).[α] D 27.8 =-20.3475 (C.0.8, CHCl 3 , 589nm), ee=96%.
Embodiment 3
[0040] Embodiment 3 prepares R-(+)-2-hydroxyl-2-(3-phenoxyphenyl) acetonitrile
[0041] Extract (R)-alcohol nitrilase (400mg) from bitter almond, 10mL of 0.1M citrate buffer solution with pH=5.4, potassium cyanide (98mg), glacial acetic acid (120mg) and 3-phenoxyphenyl Formaldehyde (198mg, 1mmol), dissolved in 10mL of isopropyl ether, was reacted for 3 days at a reaction temperature of 0°C, and R-(+)-2-hydroxyl-2-(3-phenoxyphenyl) was isolated Acetonitrile, 78% yield. 1 H NMR (CDCl 3 , 300MHz) δ=7.43-7.04(m, 9H), 6.38(s, 1H), 2.16(s, 3H).[α] D 25.8 =22.3475 (C.1.0, CHCl 3 , 589nm), ee=93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com