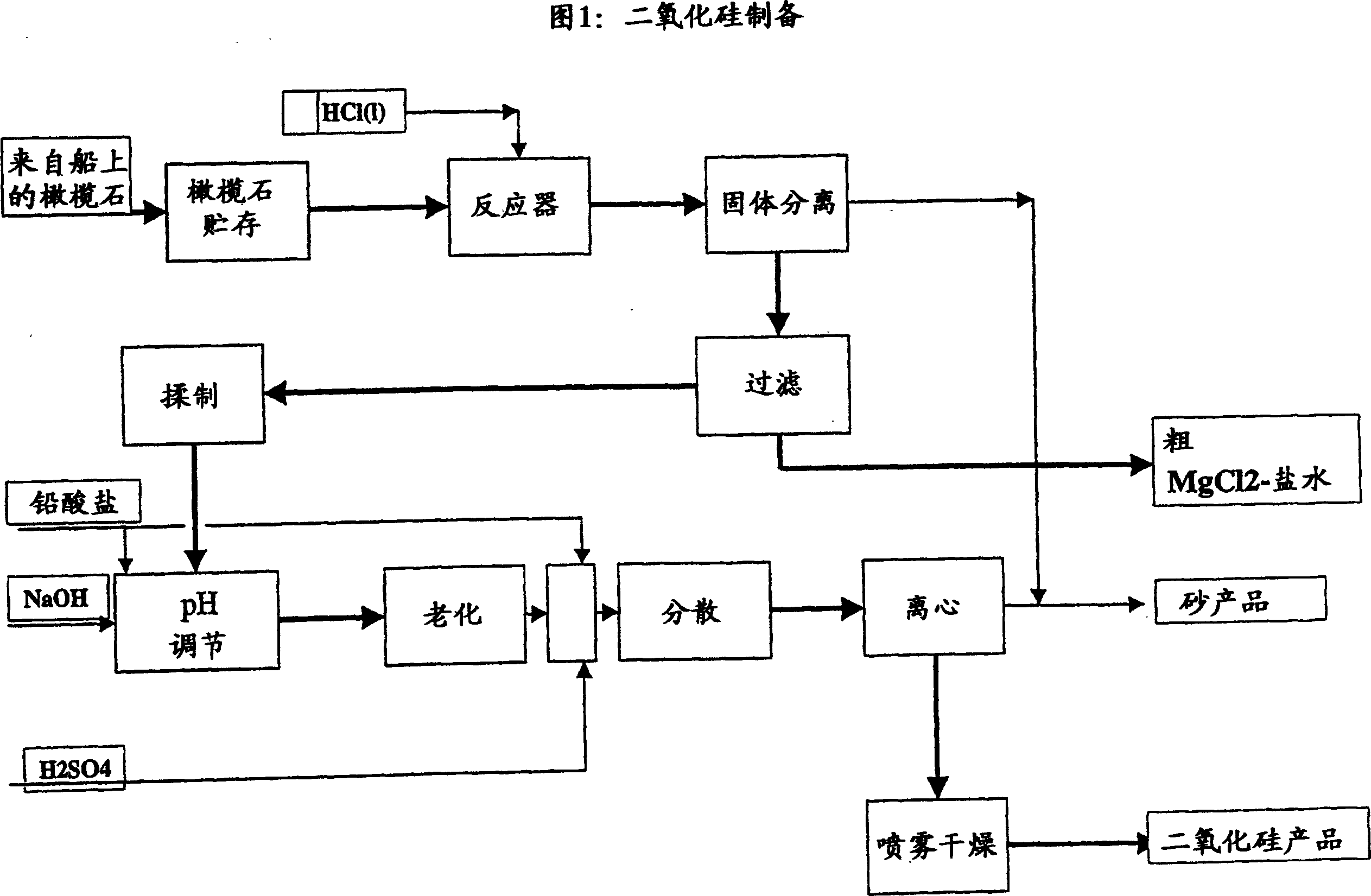
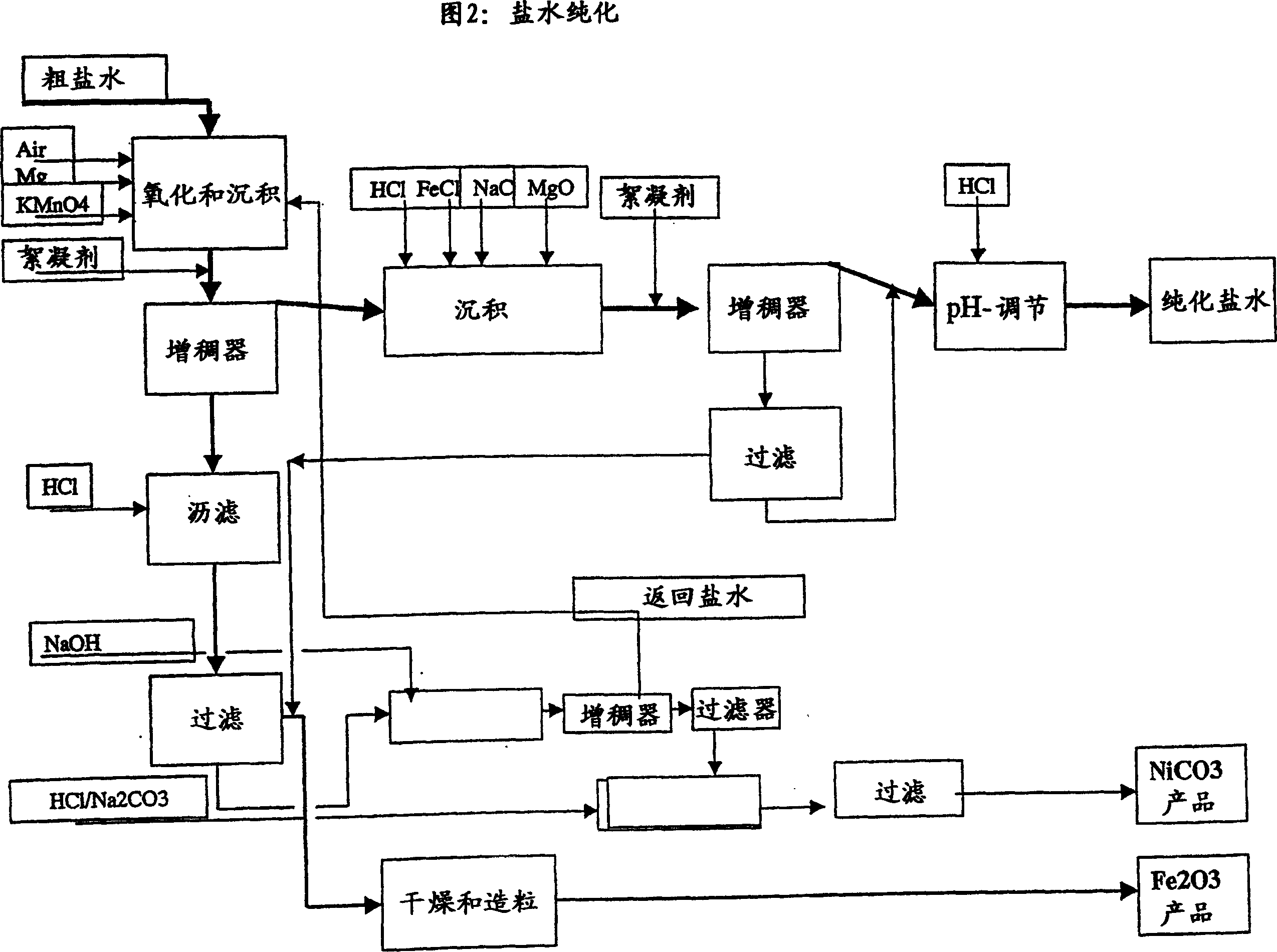
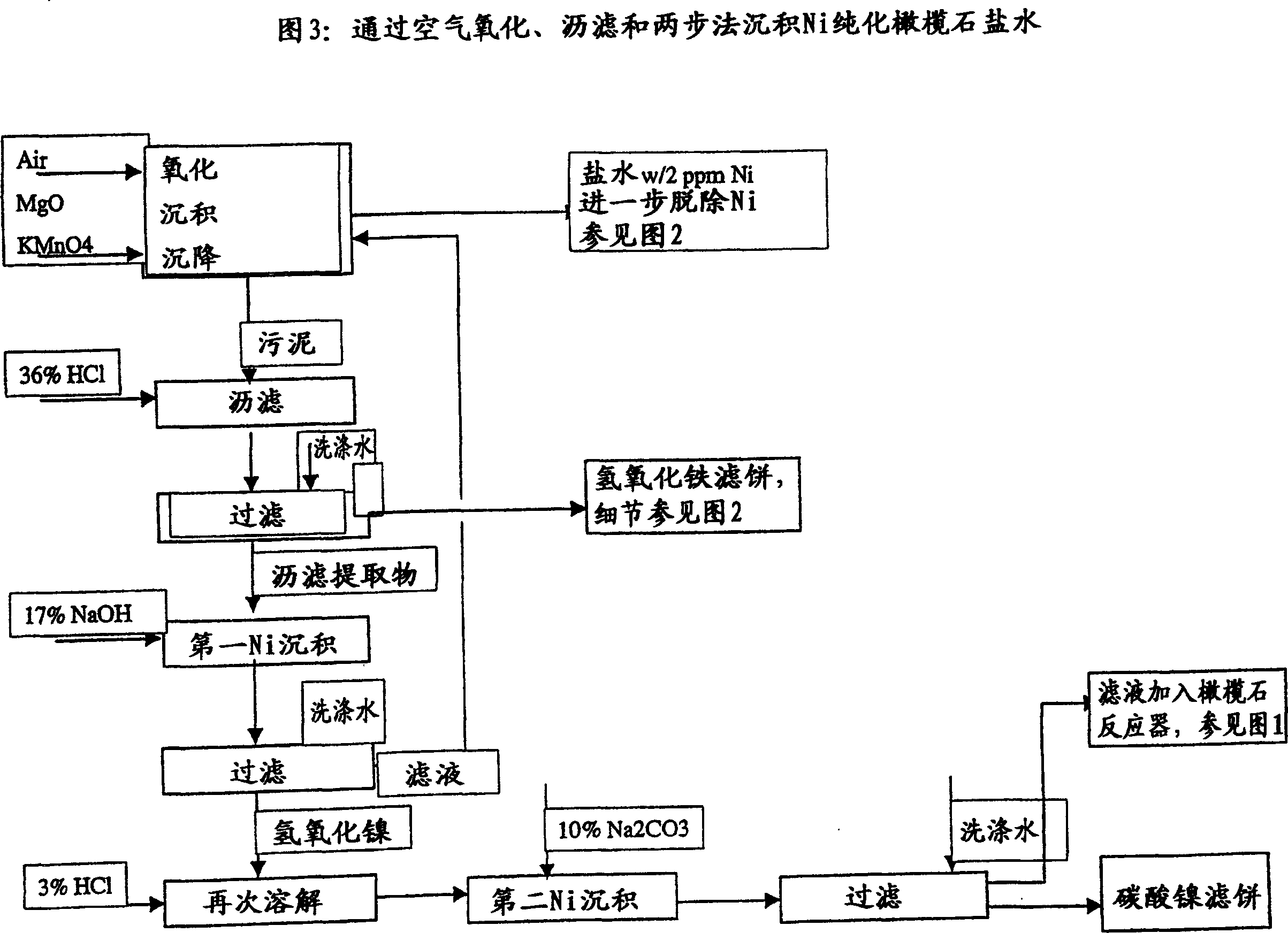
Process for complete utilisation of olivine constituents
A technology of olivine and brine, applied in chemical instruments and methods, improvement of process efficiency, inorganic chemistry, etc., can solve the problem of expensive waste treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Oxidation / deposition of iron, nickel and manganese
[0036] Treat with air containing 22% MgCl 2 , 1.0% Fe, 0.05-0.06% Ni and 1.2% HCl crude brine of olivine, and adding Mg(OH) in various proportions 2 slurry to observe the extent of elemental deposition as a function of pH. The results show that the average residence time is about 50 minutes and relative to Fe 2+ For example, when the air flow rate is 3.6 times the stoichiometric amount, ferrous iron is easily oxidized by air.
[0037] Adding just below this amount of base, relative to complete oxidation and deposition of ferric hydroxide, the main compound formed is hematite Fe 2 o 3 . As the amount of alkali increases, a more complex phase is formed, i.e. bischofersite Mg 4 Fe(OH) 8 OCl·xH 2 O (or related phase).
[0038] A significant reduction in nickel content was observed, from 0.057% Ni to less than 1 ppm Ni, and approaching the required 0.3 ppm Ni. Then the molar ratio of Fe / Ni in the depos...
Embodiment 2
[0043] Example 2: Deposition of Ni in extracts of leached Fe-Ni sludge
[0044] Prepare a batch containing 25% MgCl 2 and 0.38% Ni synthetic brine. This solution was used to carry out a continuous deposition test in two 500-ml storage tanks. The residence time for each tank was 15 minutes. Add brine and 17% NaOH to the first storage tank.
[0045] The pH (dir) of the first storage tank was varied between 5.5 and 5.8. With increasing NaOH addition, the Ni concentration in the filtered samples decreased and stabilized at about 0.02% Ni (Fig. E2.1). As the amount of alkali added increases, the Cl in the sediment tends to decrease, which indicates that Cl is replaced by OH. At the same time, the Mg content tends to increase; but it is not higher than when the washed Fe-Ni filter cake is used as a benchmark at a lower concentration of the leached extract (3% MgCl 2 and 0.15% Ni) measured values when depositing nickel hydroxide.
[0046] Figure E2.1 The relationship between...
Embodiment 3
[0055] Example 3: Deposition of Nickel Carbonate in Chloride Solution
[0056] In order to check whether nickel can be deposited as carbonate, several experiments were carried out. In some reference works, MgCO 3 and NiCO 3 The Ksp values are not much different, and it is not clear which of them is lower, i.e. which carbonate is more likely to be deposited first.
[0057] A Mg / Ni molar ratio of 0.2 can be expected by dissolving the nickel hydroxide of the first deposition process (in the 25% leach extract). In the following experiments it was intended to apply a more conservative value of 0.3 and to prepare 10 kg batches of the following composition (added as chloride): 2.0% Ni, 0.248% Mg→Mg / Ni molar ratio=0.30.
[0058] Two 500-ml reactors were placed on a magnetic stirrer, and an inlet stream of nickel solution and 10% Na 2 CO 3 , corresponding to a residence time of 27 minutes for each tank. When the storage tank was filled, a temperature of 80°C was reached. Throu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com