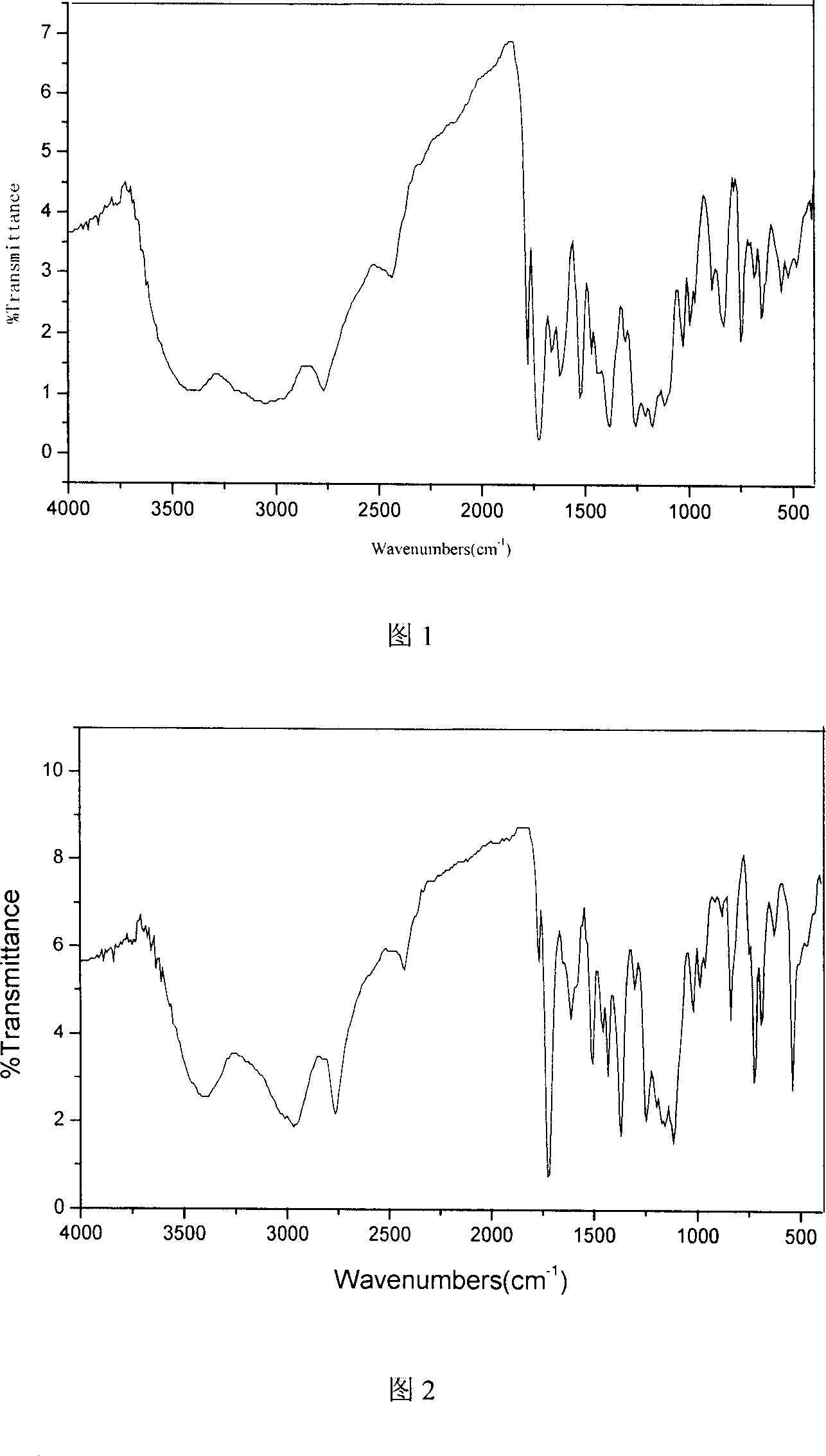
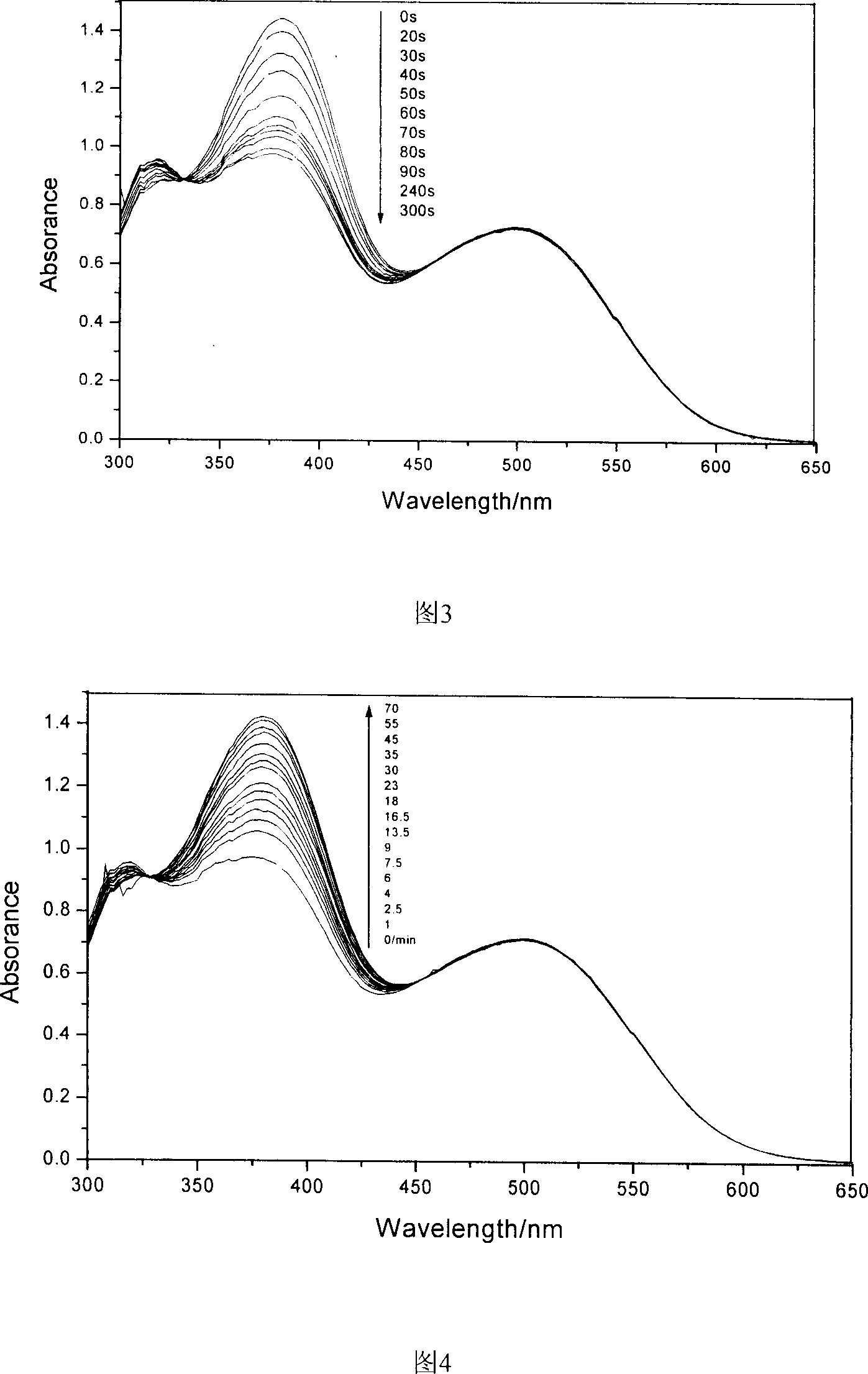
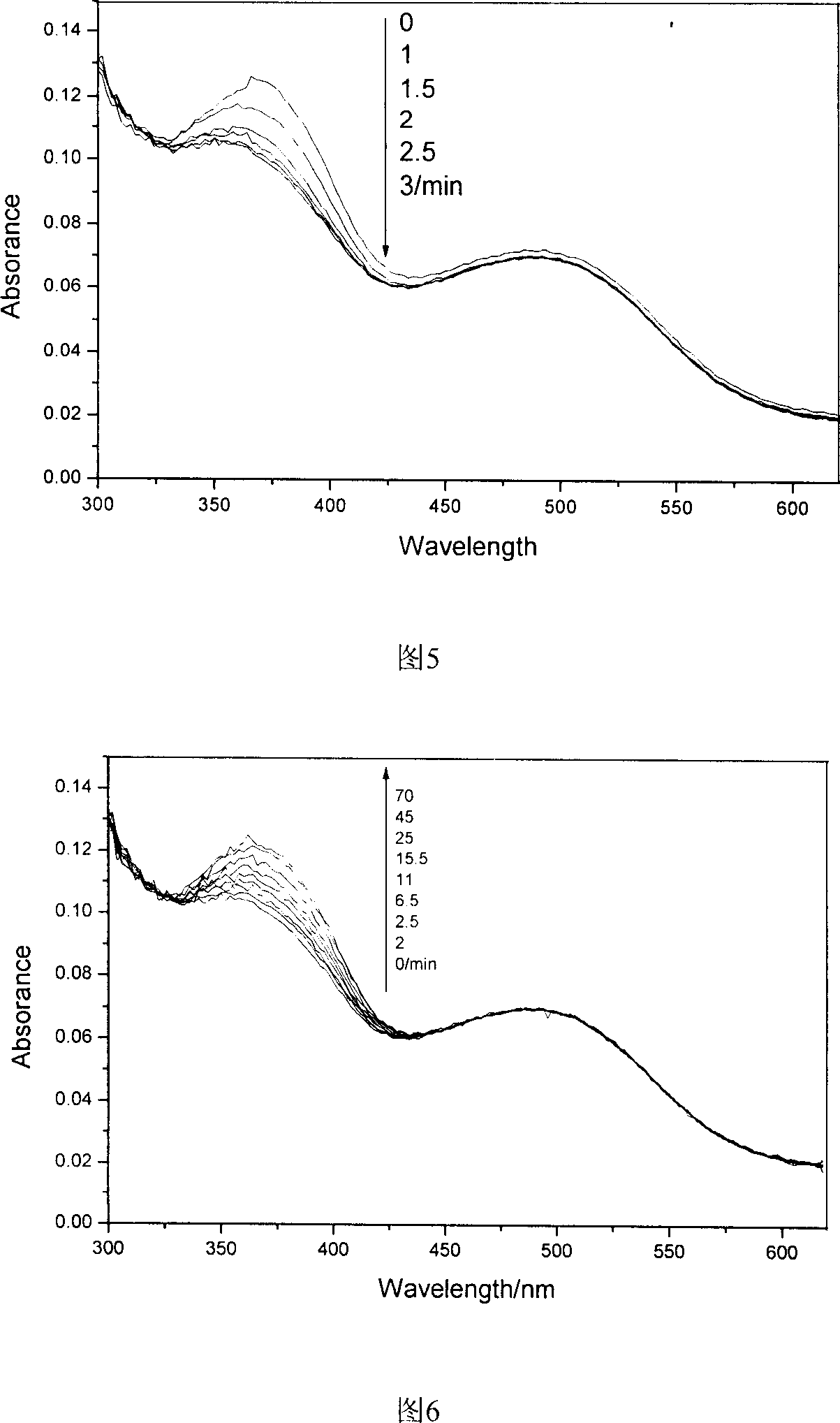
Polyimide containing fluorine, and its preparing method and use
A fluorine-containing polyimide, polyimide technology, applied in chemical instruments and methods, color-changing fluorescent materials, etc., can solve problems such as unreported photochromic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Dissolve 1.47g 3,3',4,4'-biphenyltetracarboxylic dianhydride (BPDA, Japan TCI Company) in 20g N,N-dimethylformamide (DMF), add In a four-neck flask, take 5,5'-(hexafluoroisopropyl)-bis-(2-aminophenol) (6FHP , Japan TCI company) is also dissolved in the DMF solution of 20g, adds in the above-mentioned four-necked flask after dissolving completely, at room temperature under N 2 Electromagnetic stirring in the environment for 1 hour generates a polyimide acid (PAA) solution; 2.2 ml of toluene solution is added thereto, and the polyimide acid solution is thermally cyclized at 160° C. for 2.5 hours. Water is taken out by azeotropic toluene to obtain fluorine-containing polyimide containing active hydroxyl groups; 1.57g of disperse red DR-1 containing azobenzene and 1.31g of triphenylphosphine are dissolved in 16g of DMF, and then added Add the above-mentioned fluorine-containing polyimide with active hydroxyl groups, continue to stir at room temperature for 25 hou...
Embodiment 2
[0031] Example 2 Dissolve 1.09g of pyromellitic anhydride (PMDA) in 33g of N,N-dimethylformamide (DMF), add it to a four-necked flask after completely dissolving, and take an equimolar amount of pyromellitic anhydride. 5,5'-(hexafluoroisopropyl)-bis-(2-aminophenol) (6FHP, Japan TCI Company) was also dissolved in 33g of DMF solution, and added to the above four-necked flask after complete dissolution, at room temperature next to N 2 Electromagnetically stir in the environment for 1.5h to generate a polyimide acid (PAA) solution; add 2.2ml of toluene solution to it, and thermally cyclize the polyimide acid solution at 160°C for 4h. Water is taken out by azeotropic toluene to obtain a fluorine-containing polyimide containing active hydroxyl groups; 1.26g of disperse red DR-1 containing azobenzene and 1.32g of triphenylphosphine are dissolved in 19g of DMF, and then added Add the above-mentioned fluorine-containing polyimide with active hydroxyl groups, continue to stir at room t...
Embodiment 3
[0032] Example 3 Dissolve 1.09g of pyromellitic anhydride (PMDA) in 48g of N,N-dimethylformamide (DMF), add it to a four-necked flask after completely dissolving, and take an equimolar amount of pyromellitic anhydride. 5,5'-(hexafluoroisopropyl)-bis-(2-aminophenol) (6FHP, Japan TCI Company) was also dissolved in 48g of DMF solution, and added to the above-mentioned four-necked flask after complete dissolution, at room temperature next to N 2 Electromagnetically stir in the environment for 2 hours to generate a polyimide acid (PAA) solution; add 2.2ml of toluene solution to it, and thermally cyclize the polyimide acid solution at 160°C for 5 hours, and at the same time produce water Azeotroped by toluene to obtain fluorine-containing polyimide containing active hydroxyl groups; 1.26g of disperse red DR-1 containing azobenzene and 1.32g of triphenylphosphine were dissolved in 31g of DMF, and then added to In the above-mentioned fluorine-containing polyimide with active hydroxyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com