Invert-sugar powdery injection and its production
A technology for inverting sugar and injection, which is used in medical preparations containing active ingredients, powder delivery, pharmaceutical formulations, etc. It can solve the problems of blood sugar fluctuation, inability of patients, and excessive glucose loss through urine, and achieve rapid energy and blood sugar fluctuations. Small, long-lasting effect of energy supply
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Determination of Invert Sugar Formula for Injection
[0023] 1. Invert sugar formula
[0024] Recipe (specification: 12.5g)
[0025] 1000 bottles
[0026] Fructose 6250g
[0027] Glucose 6250g
[0028] 2. Formula basis
[0029] 1) Determination of dosage form and selection of specifications
[0030] Invert sugar for injection (invert sugar for injection) is a sterile powder injection made by mixing equal amounts of glucose and fructose.
[0031] The specifications of Invert Sugar Injection that have been listed in China are: 250ml: 6.25g fructose and 6.25g glucose. The specification of fructose for injection is 12.5g; 25g. We choose the specification of 12.5g invert sugar for injection per bottle for development.
[0032] 2. Formula screening
[0033] According to the research and analysis of the clinical use of the domestically marketed products Invert Sugar Injection and Fructose for Injection, coupled with the large size of the ...
Embodiment 2
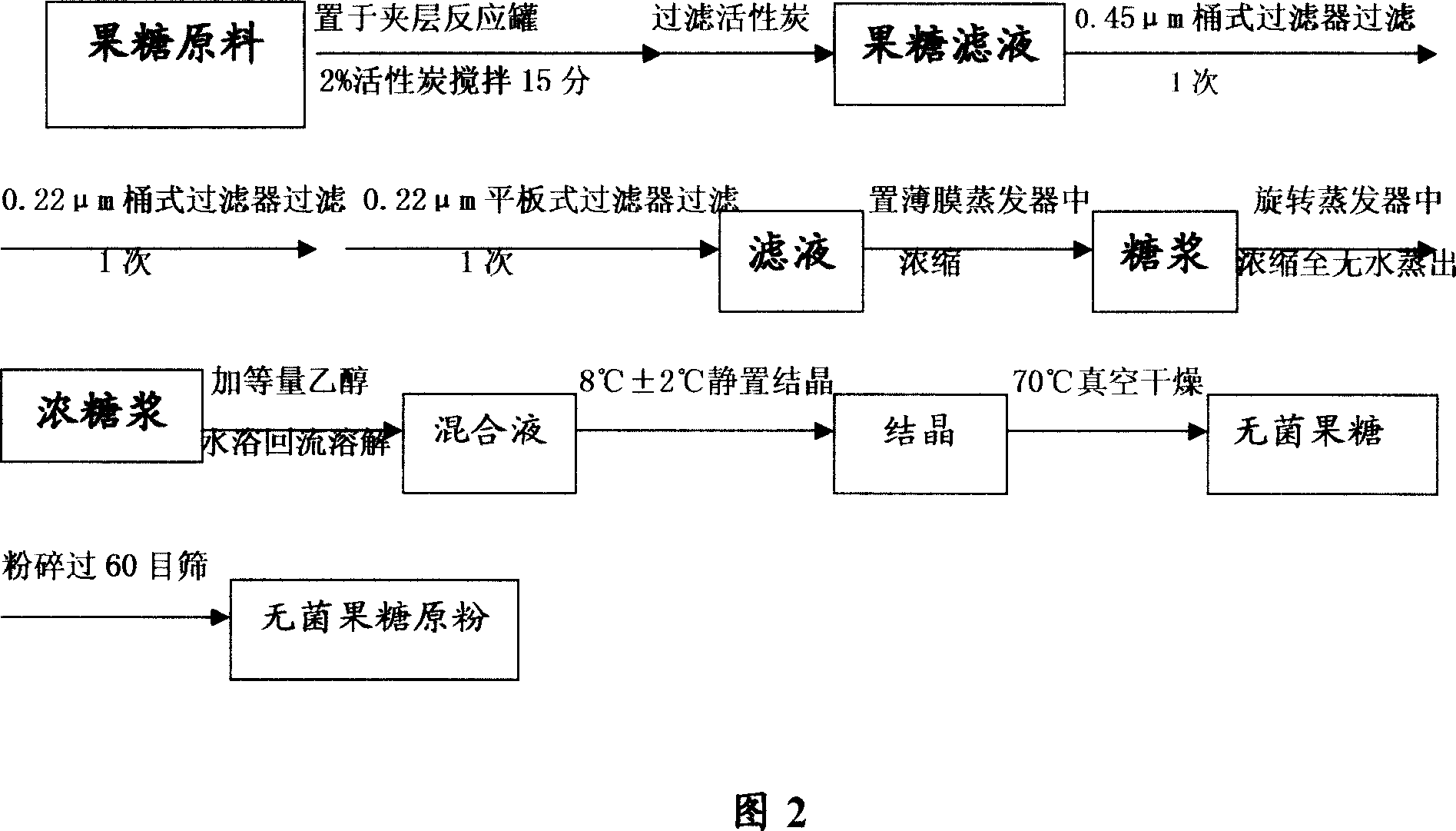
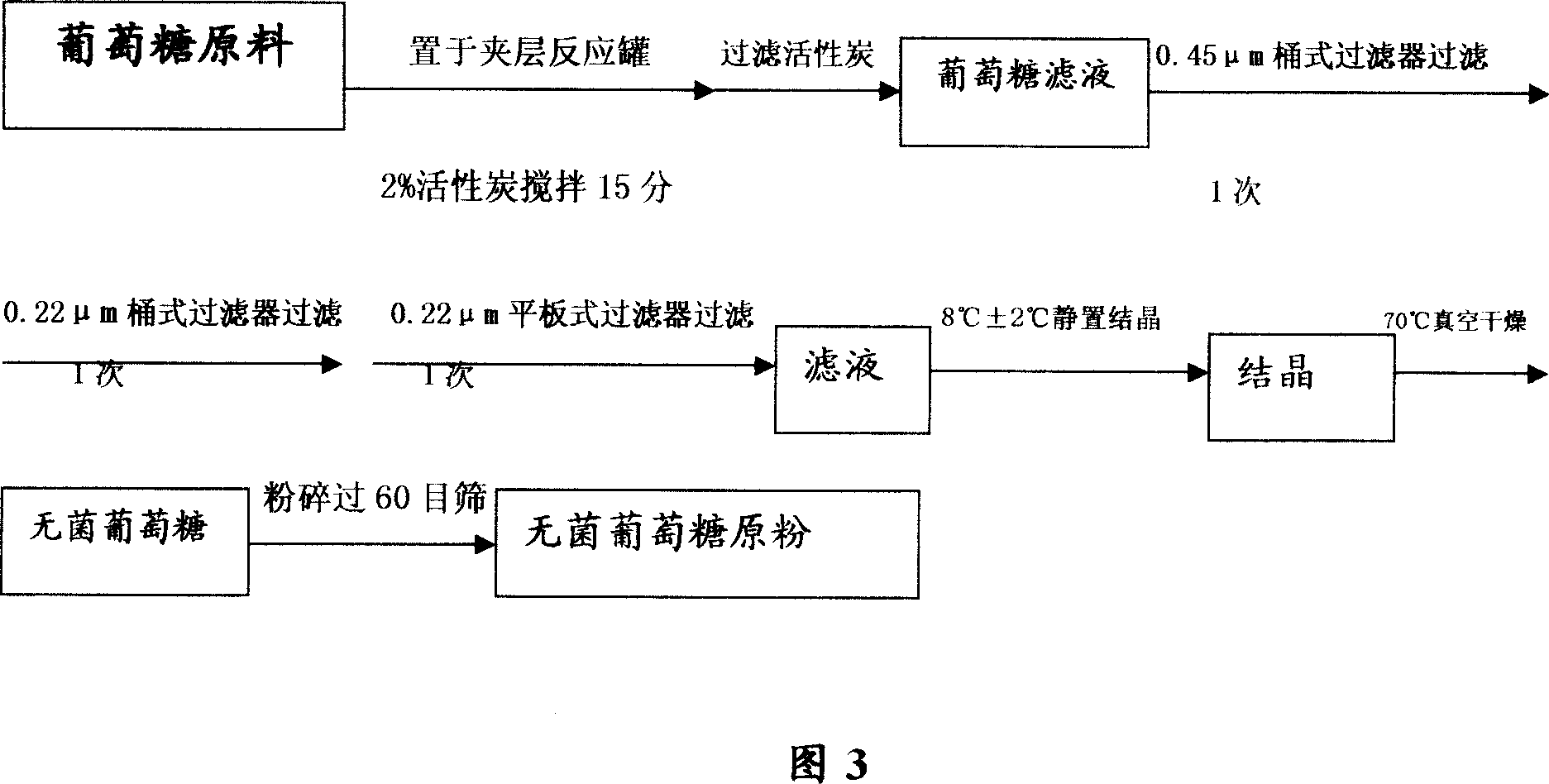
[0034] Example 2 Research on the preparation process of invert sugar for injection
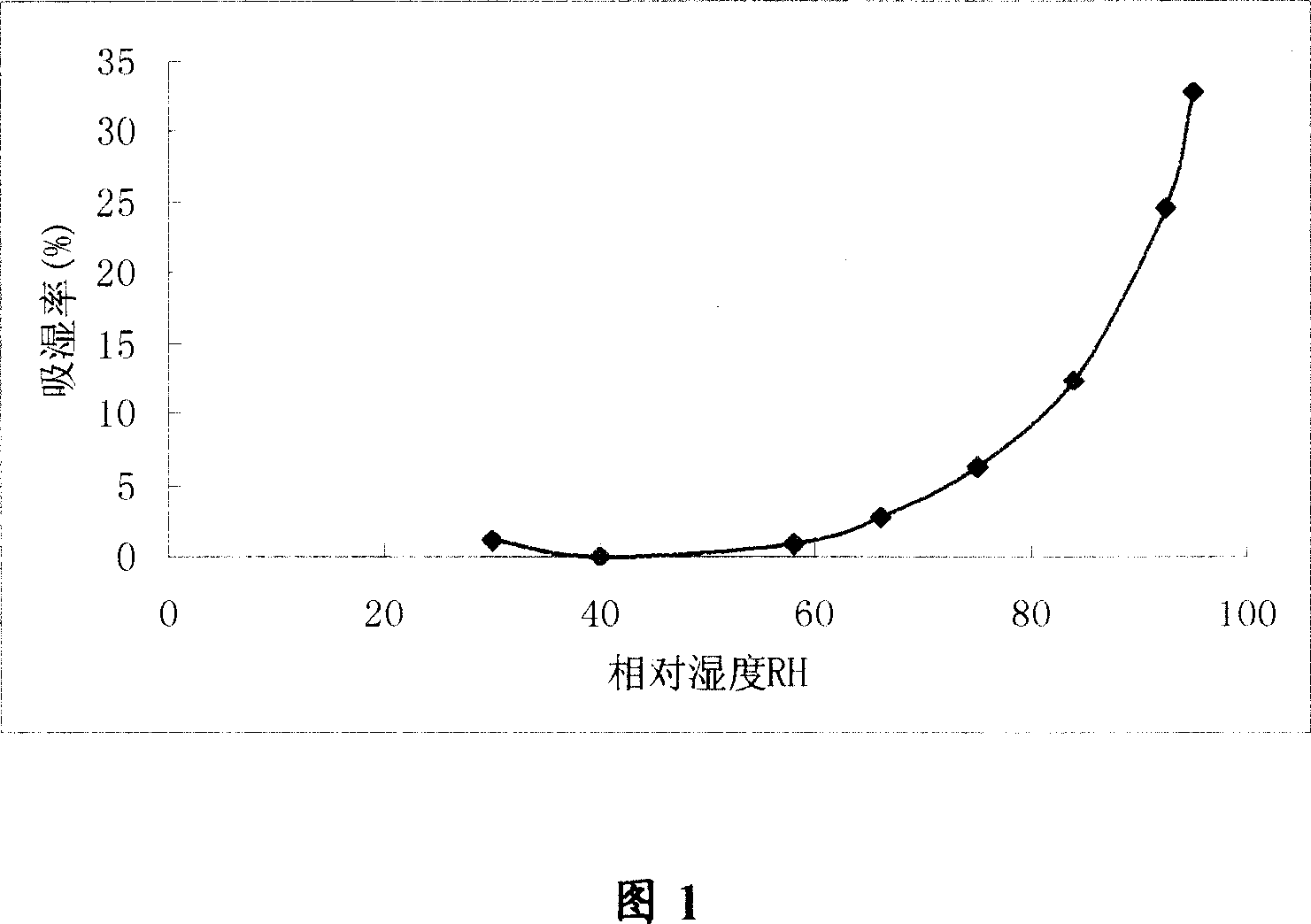
[0035] Research on physical and chemical properties
[0036] For raw materials for immediate aseptic dispensing, the requirement is applicable to the dispensing of pharmaceutical powders. First of all, the physical and chemical properties of the drug should be understood in order to develop a reasonable production process.
[0037] 1. Bulk density
[0038] Fill the invert sugar powder in the measuring cylinder, and vibrate in a certain way to ensure consistent test conditions and good reproducibility. The bulk density can be obtained from the mass and volume of the volume of the micro-powder. We measured the bulk density of invert sugar for injection (batch 040901).
[0039] Method: Put a certain amount of powder of the original medicine into a measuring cylinder, tap it gently several times, and measure its weight and volume.
[0040] Result: bulk density = 0.72 (g / ml)
[0041] Test resu...
Embodiment 3
[0074] Example 3 Test of Influencing Factors of Invert Sugar for Injection
[0075] This test was designed according to the guiding principles of drug stability testing in Appendix XI XC of Part II of the 2000 edition of the Chinese Pharmacopoeia; the test samples met the requirements of the guiding principles. Due to the three batch numbers of this product, the three specifications are obtained by aseptic subpackaging of a batch of raw materials. Therefore, we choose one specification sample of one batch number as a representative to carry out the experiment of influencing factors.
[0076] (1) Sample: batch 031201 (specification: 0.75g) test date: 2003.12.20~2004.1.5; room temperature during test: 15℃~22℃, relative humidity: 55%~80%
[0077] (2) Test items: appearance color, loss on drying, pH, solution clarity and color, pyridine and polymer inspection, related substances, sodium carbonate content, content and labeled content.
[0078] (3) Detection method: carry out acco...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Bulk density | aaaaa | aaaaa |
| Angle of repose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com