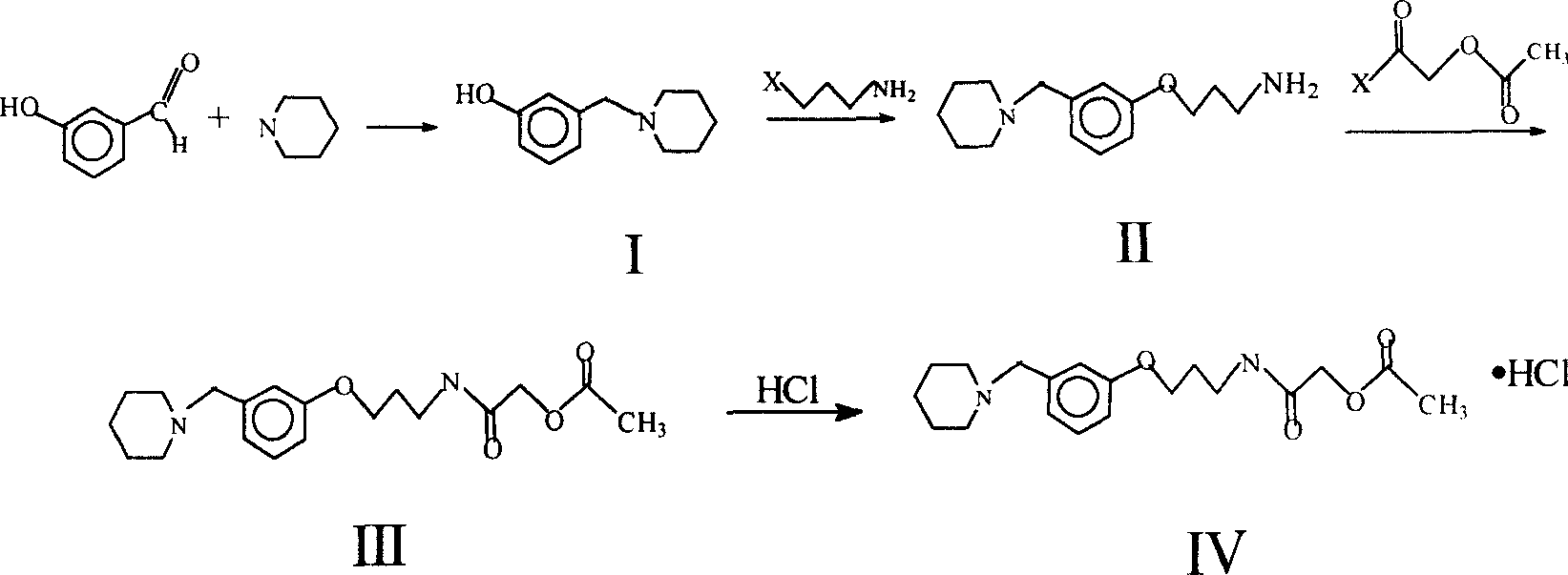
Synthesis process of roxatidine acetate hydrochloride
A technology of roxatidine acetate and a synthesis method, which is applied in directions such as organic chemistry, can solve the problems of low purity of the target compound, complicated operation, difficult application and the like, and achieves a technology that is beneficial to industrial development and application, improves the purity, and has a simple synthesis process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Synthesis of 3-piperidylmethylphenol
[0028] Add 20g of m-hydroxybenzaldehyde, 110ml of methanol, and 34g of piperidine into a three-necked flask, and stir at room temperature for 0.5 hours to dissolve completely. Add 8.8g KBH within 1 hour 4 , continue to stir at room temperature for 1 hour, the reaction resultant is concentrated under reduced pressure, is cooled to room temperature, adds 200ml 3N hydrochloric acid to dissolve, extracts with ethyl acetate, ammoniacal liquor basifies the aqueous layer, separates out white solid, filters, and can obtain target object (I ) 27g. m.p.135℃~138℃.
Embodiment 2
[0030] Synthesis of 3-piperidylmethyl-phenoxypropylamine
[0031] 20g of 3-chloropropylamine was dissolved in 45ml of 3N NaOH solution (containing 10% NaCl), and extracted with 350ml of toluene.
[0032] Add 19g of 3-piperidinylmethylphenol, 4.5g of NaOH, 45ml of DMSO, and 25ml of toluene into a three-necked flask with a water separator, reflux at 110°C to separate the water, and after no water is removed, add the 3-chloropropylamine toluene extract, and complete the addition Afterwards, reflux at 110°C to 120°C for 4 hours. After completion, NaCl was filtered off, and the filtrate was concentrated under reduced pressure. Add the mixed solvent of 400ml ethanol-acetone-ethyl acetate (volume ratio=5:2:3), dropwise add 2-methylsuccinic acid-ethanol saturated solution therein, separate out white powder, filter out and add water to dissolve, use 0.8NNaOH solution adjusts the pH value between 6.0 and 8.0, extracts impurities with toluene, then uses 1.5NNaOH solution to adjust the p...
Embodiment 3
[0034] Synthesis of Acetoxyacetyl Chloride
[0035] Dissolve 35g of glycolic acid in 50ml of DMF, add 36g of acetyl chloride dropwise with stirring at room temperature, heat up to 50°C for 1.5 hours after the dropwise addition, concentrate the reaction solution under reduced pressure, and add chloroform-SOCl to the concentrate 2 The solution was heated up to 50°C with stirring, kept for 2 hours, then distilled under reduced pressure, and fractions at 67°C to 77°C / 1kPa were collected.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com