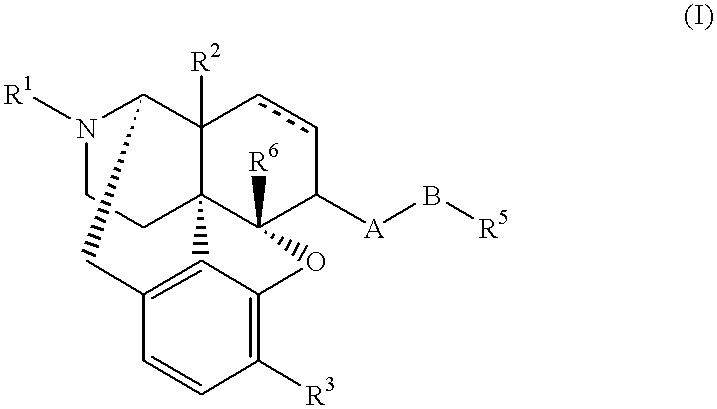
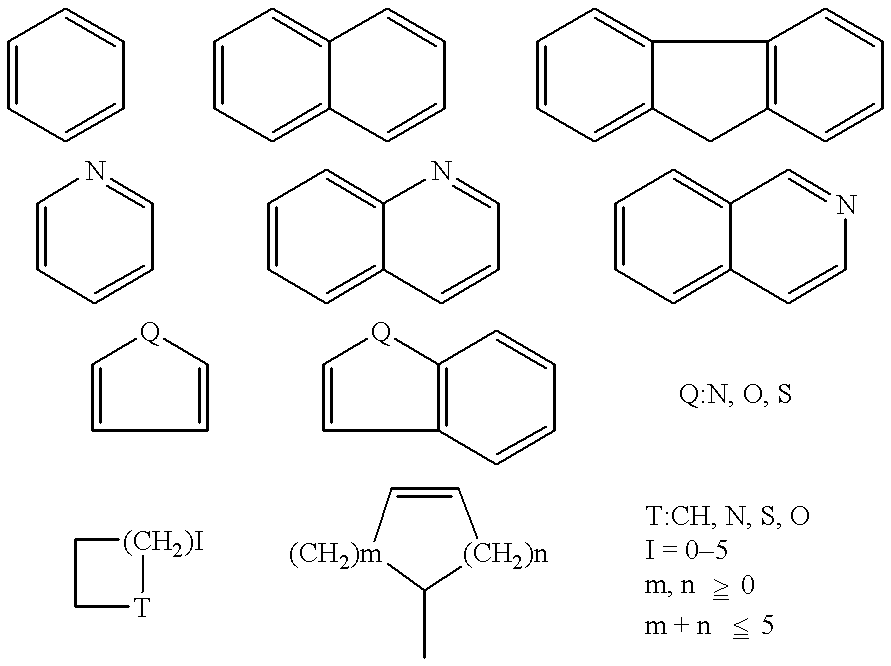
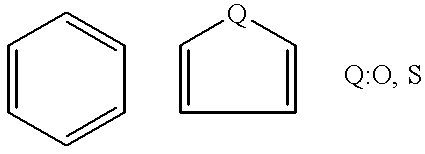
Stable medicinal compositions containing 4,5-epoxymorphinane derivatives
a technology of epoxymorphinane and stable composition, which is applied in the direction of drug compositions, aerosol delivery, inorganic non-active ingredients, etc., can solve the problems of undetermined stabilization method, 4,5-epoxymorphinan derivatives are chemically unstable to heat, light and oxygen, and the stability of compositions has not been determined. , to achieve the effect of stabilizing external preparations and achieving greater stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
(Prescription Example 1)
Injection
[0053]
4TABLE 3 Drug Isotonizing Additive Example 2 Dosage Form Content Agent Rate Comparative Injection 10 .mu.g / mL Sodium chloride 0.90% Example Test 1 Injection 10 .mu.g / mL Glucose 5.00% Test 2 Injection 10 .mu.g / mL Xylitol 5.00% Test 3 Injection 10 .mu.g / mL Mannitol 5.00% Test 4 Injection 10 .mu.g / mL D-sorbitol 5.00%
[0054] Stability test: After the aqueous solutions of Tests 1 to 4, and Comparative Example were subjected to nitrogen bubbling, they were sealed in ampoules. Then, after storage at a temperature of 80.degree. C. for seven days, the residual rates of Compound 1 were measured using a HPLC method (an UV method). Thus, stability after accelerated storage was estimated.
5TABLE 4 Isotonizing Storage Residual Example 2 Agent Additive Rate Conditions Rate (%) Comparative Sodium 0.90% Seal / 80.degree. C. / 7d 66.6 Example chloride Test 1 Glucose 5.00% Seal / 80.degree. C. / 7d 90.3 Test 2 Xylitol 5.00% Seal / 80.degree. C. / 7d 97.9 Test 3 Mannitol 5.00% ...
example 2
(Prescription Example 2)
Injection
[0057]
7TABLE 5 Additive Example 3 Dosage Form Drug Content Antioxidant Rate Comparative Injection 10 .mu.g / mL None Example Test 1 Injection 10 .mu.g / mL Sodium 0.10% thiosulfate Test 2 Injection 10 .mu.g / mL Sodium 0.50% thiosulfate Test 3 Injection 10 .mu.g / mL Sodium 1.00% thiosulfate
[0058] Stability test: The aqueous solutions of Tests 1 to 3, and that of Comparative Example were sealed in ampoules. Then, after sterilization by heating at a temperature of 120.degree. C. for 60 minutes, the purities of Compound 1 in the samples were measured using a HPLC method (an UV method). Thus, pharmaceutical stability after sterilization was estimated.
8TABLE 6 Additive Purity Example 3 Antioxidant Rate Storage Conditions (%) Comparative None Seal / 120.degree. C. / 60 min 98.95 Example Test 1 Sodium 0.10% Seal / 120.degree. C. / 60 min 99.57 thiosulfate Test 2 Sodium 0.50% Seal / 120.degree. C. / 60 min 99.44 thiosulfate Test 3 Sodium 1.00% Seal / 120.degree. C. / 60 min 99.53 ...
example 3
(Prescription Example 3)
Granule
[0061]
9 Compound 1 100 mg Sodium thiosulfate 0 to 1 g Avicel PH-101 31 g Lactose Balance volume Total 100 g
[0062]
10TABLE 7 Dosage Additive Example 4 Form Drug Content Antioxidant Rate Comparative Granule 100 .mu.g / 100 mg None Example Test 1 Granule 100 .mu.g / 100 mg EDTA 0.10% Test 2 Granule 100 .mu.g / 100 mg Citric acid 0.10% Test 3 Granule 100 .mu.g / 100 mg Propyl gallate 0.10% Test 4 Granule 100 .mu.g / 100 mg Butyl 0.10% Hydroxyanisole Test 5 Granule 100 .mu.g / 100 mg Tocopherol 0.10% Test 6 Granule 100 .mu.g / 100 mg Sodium 0.10% thiosulfate Test 7 Granule 100 .mu.g / 100 mg Sodium 0.20% thiosulfate Test 8 Granule 100 .mu.g / 100 mg Sodium 0.50% thiosulfate Test 9 Granule 100 .mu.g / 100 mg Sodium 1.00% thiosulfate
[0063] Stability test: Immediately after manufacturing the granules of Tests 1 to 9 and that of Comparative Example, the purities of Compound 1 were measured using a HPLC method (an UV method). Thus, pharmaceutical stability was estimated.
11TABLE 8 Ad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com