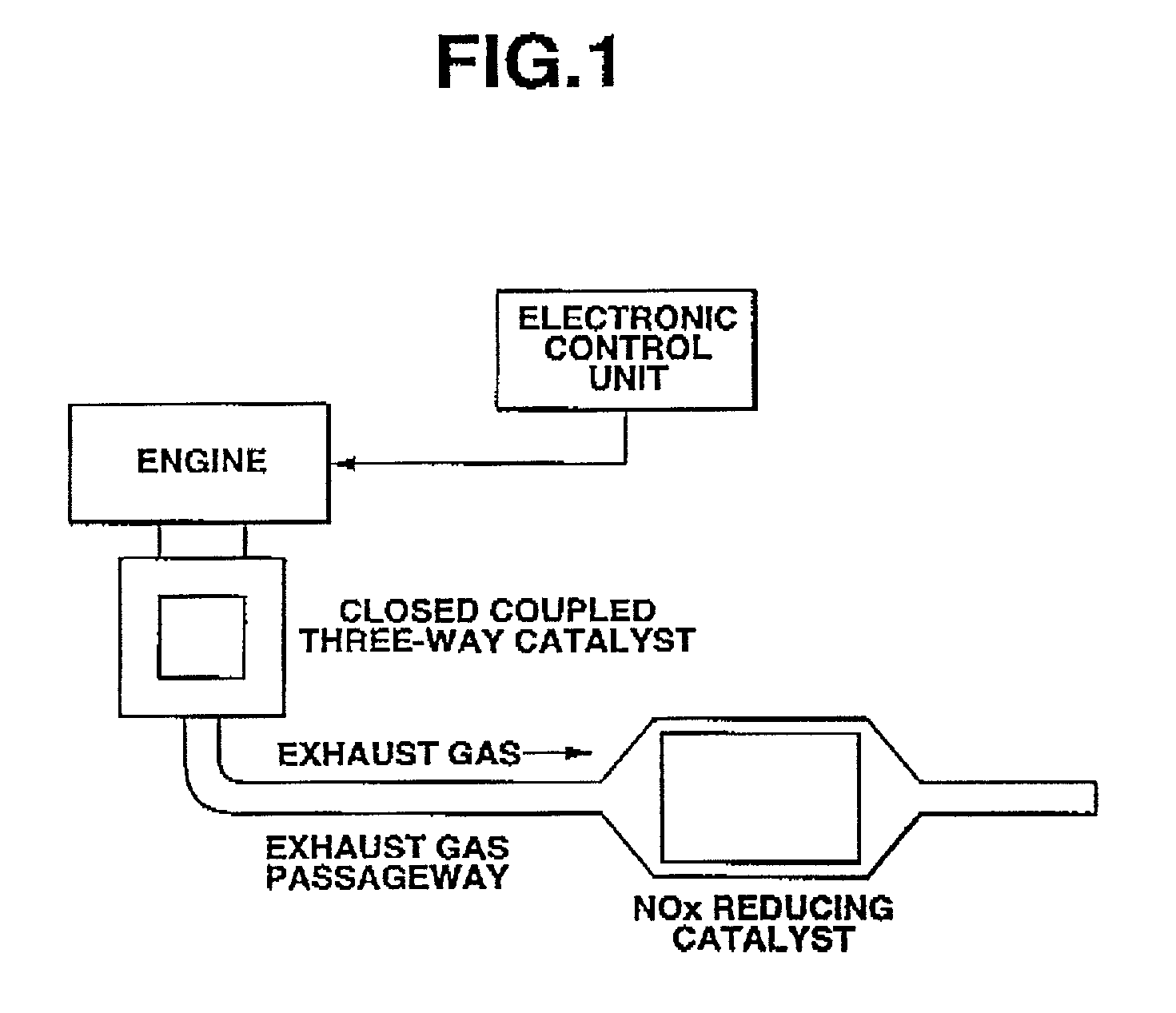
Exhaust gas purifying system and catalyst
a technology of exhaust gas purification system and catalyst, which is applied in the direction of physical/chemical process catalyst, metal/metal-oxide/metal-hydroxide catalyst, and separation process, etc. it can solve the problem that the catalytic activity (for oxidation) of platinum and rhodium cannot be sufficiently demonstrated, and the catalyst cannot reduce nox
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
(A) Preparation of a Closed Coupled Three-way Catalyst HC and CO Reforming Devices)
[0052] Pt, Pd and Rh (noble metals) and Ce and Zr (added components) were carried on activated alumina powder thereby to prepare catalytic powder. The catalytic powder was formed into slurry. This slurry was coated on a ceramic honeycomb-type monolithic substrate having a volume of 1.3 liters and formed with a plurality of axially extending cells each of which is defined by axially extending thin walls, so that the walls of the cells were coated with the slurry. Thereafter, the coated monolithic substrate was blown with air stream to remove excessive slurry in the cells, and dried at 130.degree. C. and then fired at 400.degree. C. for 1 hour. As a result, a closed coupled three-way catalyst was obtained carrying the noble metals in a total amount of 500 g per one cubic feet of the monolithic substrate, in which a weight ratio of Pt / Pd / Rh was 1 / 1 / 100 / 1 / 4. The closed coupled three-way catalyst was desig...
example 2
[0061] A closed coupled catalyst was prepared in the same manner as that in Example 1.
[0062] A NOx reducing catalyst was prepared by repeating the procedure for preparation of that in Example 1 with the exception that 15 g of Cs was carried per one liter of the monolithic substrate.
[0063] An exhaust gas purifying system of Example 2 was constructed similarly to that in Example 1.
example 3
[0064] A closed coupled catalyst was prepared in the same manner as that in Example 1.
[0065] A NOx reducing catalyst was prepared by repeating the procedure for preparation of that in Example 1 with the exception that the catalyst C was further impregnated with barium acetate and magnesium acetate so that 10 g of Ba (calculated as oxide) and 5 g of Mg (calculated as oxide) were carried per one liter of the monolithic substrate.
[0066] An exhaust gas purifying system of Example 3 was constructed similarly to that in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Angle | aaaaa | aaaaa |
| Angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com