Suppression of inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
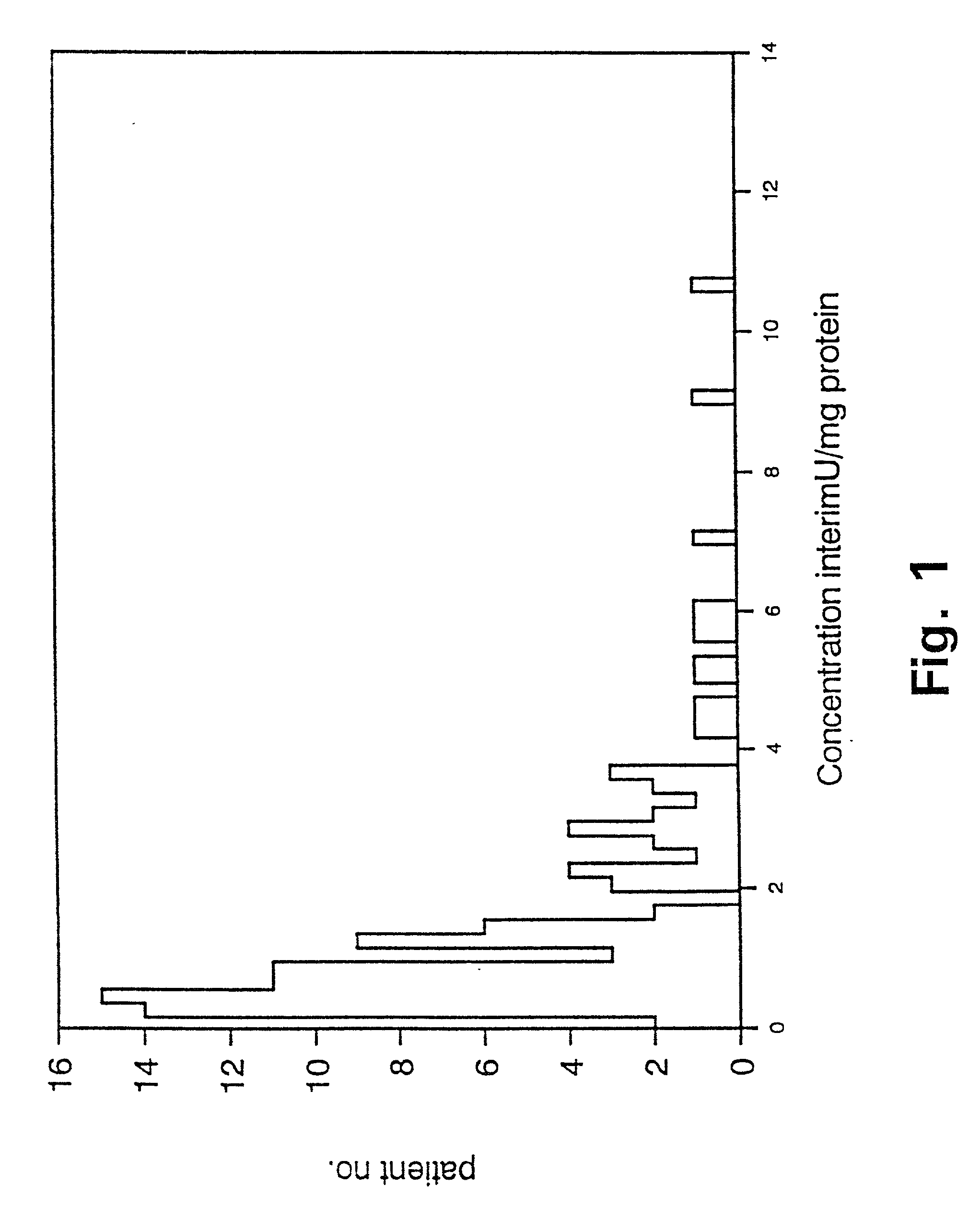
[0103] Prognostic value of PAI-1 in bronchiogenic adenocarcinomas.
[0104] In non small cell lung cancer the stage of disease, including tumour size and lymph node involvement, within the specific stages, have been reported to be among the strongest prognostic factors (62,63). Even though patients with bronchogenic adenocarcinoma are surgical radically resected (stage I and II), the 5 year survival is poor, with the 5 year survival being 40% in stage I and 10% in stage II (64). Thus a means to characterize and define patients with bronchogenic adenocarcinoma and poor prognosis is needed, to select sub-groups of radically resected patients for adjuvant chemotherapy. To our knowledge there is no such prognostic marker used in clinical practice today.
[0105] We have retrospectively studied the prognostic significance of uPA and PAI-1 levels as determined by ELISA in tumour extracts from patients with bronchogenic adenocarcinoma. The study includes 106 patients, all having had surgery with...
example 2
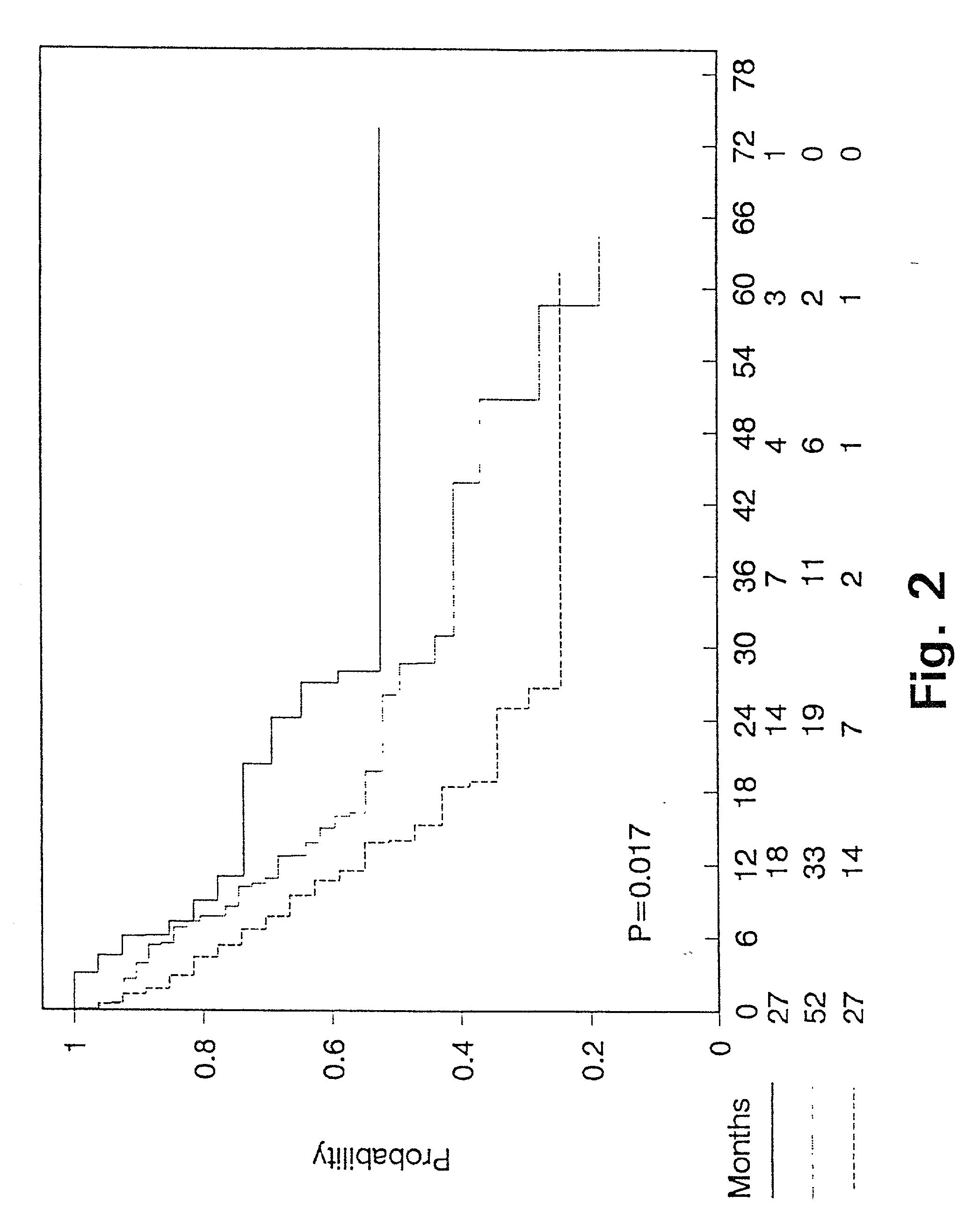
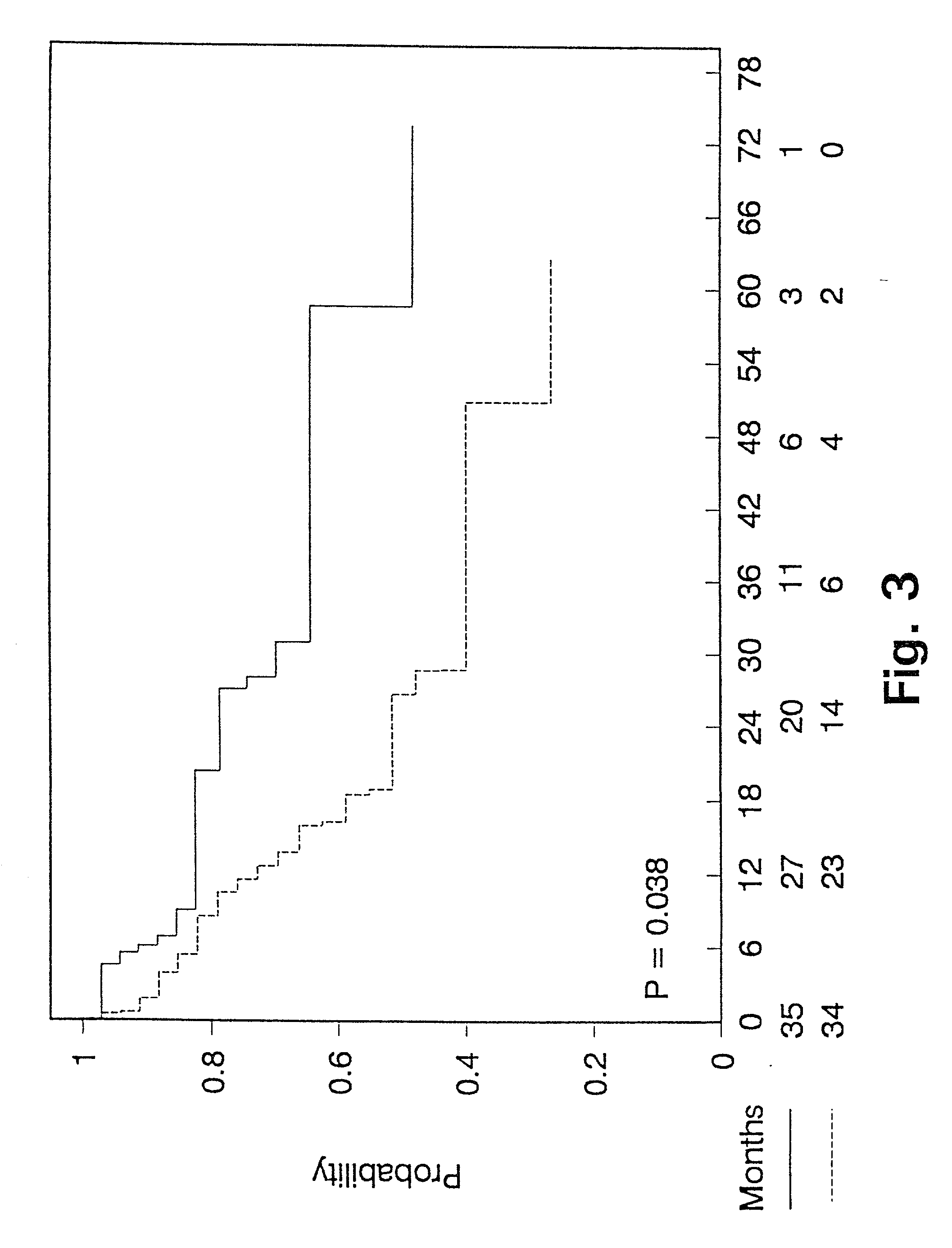
[0136] Predictive value of PAI-1 measurements in bronchiogenic adenocarcinomas.
[0137] As shown in Example 1, a proportion of lung cancer patients have high levels of PAI-1 in their tumours which predict a poor prognosis. Accordingly, patients with high PAI-1 levels will be candidates for a treatment aiming at inhibiting the binding of PAI-1 to uPA. This example describes a predictive assay to identify patients who potentially will benefit from such a treatment.
[0138] Material and Methods
[0139] Patients
[0140] Patients with bronchiogenic adenocarcinoma stage I or II referred for adjuvant therapy subsequent to radical resection of their lung tumours.
[0141] Tumour Extraction.
[0142] Tumour tissue from the patients with bronchogenic adenocarcinoma is stored at -80.degree. C. Tumour extracts are made by a procedure including pre-cooling the tissue in liquid nitrogen, mechanical pulverization, extraction with a buffer consisting 75 mM potassium acetate, 0.3 M NaCl, 0.1 M L-arginine, 10 mM E...
example 3
[0147] In situ Hybridization for PAI-1 mRNA and Immunostaining of PAI-1 Protein in Human Lung Cancer
[0148] Immunohistochemistry
[0149] Cryostat sections from 24 samples of pulmonary carcinoma were used for immunohistochemistry. These included 14 cases of squamous cell carcinoma, 5 cases of adenocarcinomas, 3 cases of mixed tumours (adenocarcinoma and squamous cell carcinoma) and 3 cases of large cell undifferentiated carcinoma. Adjacent normal mucosal tissue resected at time of surgery was obtained from 3 of the patients. Monoclonal anti-human PAI-1 antibodies (clones 1 and 2) were used for immunohistochemical stainings. An irrelevant monoclonal antibody (m-anti-TNP) was used as a negative control. The two monoclonal antibodies have previously been shown to be directed against two different epitopes on PAI-1. 5 .mu.m cryostat sections were subjected to a conventional APAAP (Alkaline Phosphatase-Anti-Alkaline Phosphatase) method as described previously (45).
[0150] In situ Hybridizatio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Cytotoxicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com