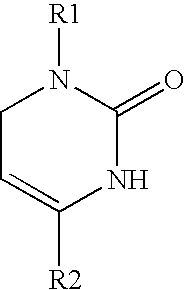
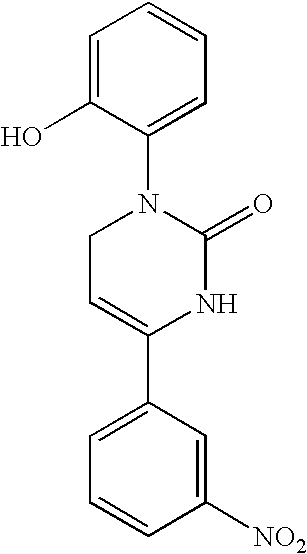
1,2,3,6-tetrahydropyrimidine-2-one compositions and therapeutic methods therewith for gastrointestinal dysfunction
a technology of pyrimidine and composition, applied in the field of gastrointestinal dysfunction, can solve problems such as local irritation at high concentrations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example a
[0055] Chemical Synthesis of Icilin and Analogs. The methods of chemical synthesis are as described by Podesva and Do Nascimento in U.S. Pat. No. 3,821,221, herein incorporated by reference. Briefly, a substituted acetophenone, e.g. 3-nitroacetophenone or 3-trifluoromethylacetophenone, readily obtainable from commercial sources such as Sigma-Aldrich, Co., was mixed with diethylamine or dimethylamine in formaldehyde and refluxed in acidic solutions. After addition of a second substituent (e.g. ortho-aminophenol), the Mannich reaction produced a .beta.-amino-ketone compound (e.g. [.beta.-ortho-hydroxyanilino]-meta-nitropropiophenone) which was isolated. This reagent was then reacted with potassium cyanate or sodium cyanate to produce an unstable urea intermediate that proceeds to cyclize into the tetrahydropyridimine-2-one ring, with the appropriate groups on position 1 (2-hydroxyphenyl) and 4 (3-nitrophenyl) on the 1,2,3,6-tetrahydropyrimidine-2-one ring. The precipitated product was...
example 1
[0056] A male subject spread 10 mg of icilin with a swab-stick between two sticks of a fruit-flavored chewing gum and chewed the gum for 10 minutes. No sensations of coolness were noted in the mouth or throat. But after 10 minutes general sensations of coolness were felt in the retrostemal and epigastric areas. These cooling effects lasted for about 1 hour. By contrast, the chewing of mentholated gum or mentholated candy produced, after an initial harsh taste, strong cooling of the mouth and throat that lasted only about 10 minutes. The next day the subject consumed a quick meal consisting of a large-size pepperoni-sausage pizza, washed down with coca-cola. The subject reported feelings of satiety, bloating, and severe epigastric discomfort. Consumption of 5 mentholated candies (Mentos) did not affect these symptoms. However, using the icilin-chewing gum reduced all symptoms.
example 2
[0057] A female subject who met the diagnostic criteria for gastroesophageal reflux disease (GERD) would, on occasions after a big meal, wake in the middle of night with severe epigastric discomfort, eructations, a sour taste in the mouth, and a sense of nausea and pain. Ingestion of Tagamet.RTM. would provide some degree of relief from pain, but the other symptoms persisted. The subject was given the icilin-chewing gum wafer and instructed on its use. She reported that using the icilin-chewing gum on two different evenings helped relieve her discomfort and allowed her to go to sleep uneventfully.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com