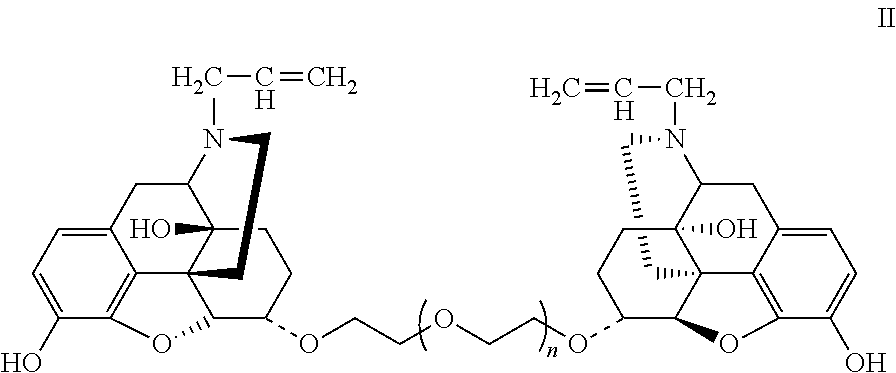
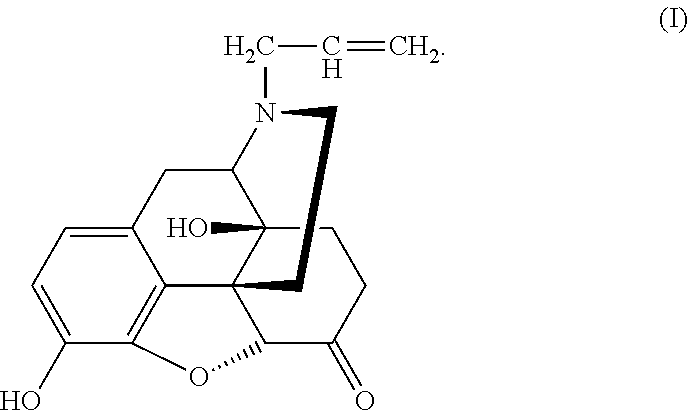
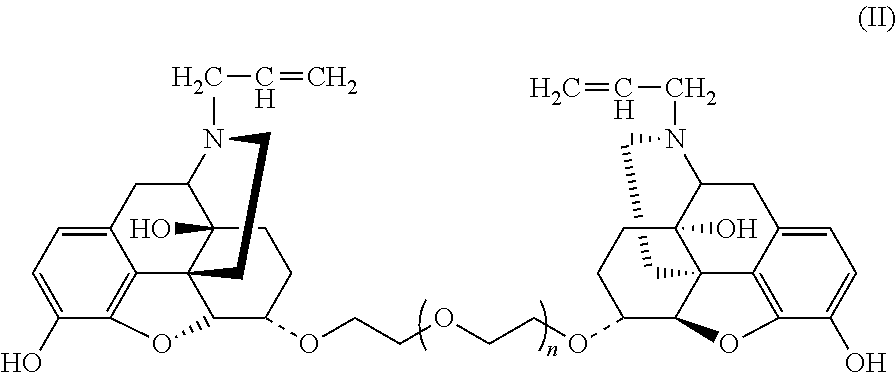
Conjugate of Polyethylene Gylcol and Naloxone and Pharmaceutical Composition and Use Thereof
a technology of polyethylene gylcol and naloxone, which is applied in the field of hydrophilic polymernaloxone conjugate, can solve the problems of side effects that affect the quality of life of patients, ineffective orally administered in the form of tablets, and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Double-End Substituted Hexaethylene Glycol-Naloxone (α,α)-NAL26 and (β,β)-NAL26 (Compounds E2 and E1, Wherein f=6)
[0031]
Synthesis of Compound B:
[0032]6 g Naloxone hydrochloride A was added into a round bottom flask and dissolved in 30 ml dichloromethane. 11.7 g di-isopropylethylamine (DIPEA) was added after complete dissolution of Naloxone hydrochloride A. The mixture was stirred under nitrogen for 15 min before 7.6 g MEMC1 was added slowly and dropwise into the solution. Then reaction was allowed at room temperature for 24 h. After complete reaction monitored by TLC, stirring was stopped, and water was added three times (30 ml×3) for extraction. The organic phase was washed once with a saturated sodium chloride solution before it was dried over anhydrous sodium sulfate. Concentration and purification by column chromatography offered 5.37 g Compound B with a yield of 87%.
Synthesis of Compound C1 (α Configuration) and Compound C2 (β Configuration):
[0033]5.37 g Compound B...
example 2
Synthesis of Double-End Substituted Tetraethylene Glycol-Naloxone (α,α)-NAL24 (Compound E2, Wherein f=4)
[0039]Except that tetraethylene glycol was used instead of hexaethylene glycol, the procedure of Example 1 was repeated to obtain the double-end substituted tetraethylene glycol-naloxone (α,α)-NAL24. m / z [MH]+ 817. 1H-NMR (CDCl3): 1.14-1.24 (m, 4H), 1.46-1.58 (m, 8H), 2.1-2.23 (m, 4H), 2.52-2.62 (m, 4H), 2.89 (d, 2H), 3.00-3.11 (m, 6H), 3.55-3.78 (m, 16H), 3.86-3.89 (m, 2H), 4.72 (d, 2H), 5.15-5.21 (m, 4H), 5.77-5.85 (m, 2H), 6.50 (d, J=8.16 Hz, 2H), 6.71 (d, J=8.1 Hz, 2H).
example 3
Synthesis of Double-End Substituted Dodecaethylene Glycol-Naloxone (α,α)-NAL212 (Compound E2, Wherein f=12)
[0040]Except that H(OCH2CH2)12—OH was used instead of hexaethylene glycol, the procedure of Example 1 was repeated to obtain the double-end substituted dodecaethylene glycol-naloxone (α,α)-NAL212. m / z [MH]+ 1169. 1H-NMR (CDCl3): 1.12-1.24 (m, 4H), 1.45-1.589 (m, 8H), 2.11-2.24 (m, 4H), 2.50-2.61 (m, 4H), 2.89 (d, 2H), 3.02-3.11 (m, 6H), 3.50-3.78 (m, 48H), 3.86-3.90 (m, 2H), 4.71 (d, 2H), 5.14-5.20 (m, 4H), 5.75-5.85 (m, 2H), 6.51 (d, J=8.17 Hz, 2H), 6.70 (d, J=8.1 Hz, 2H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com