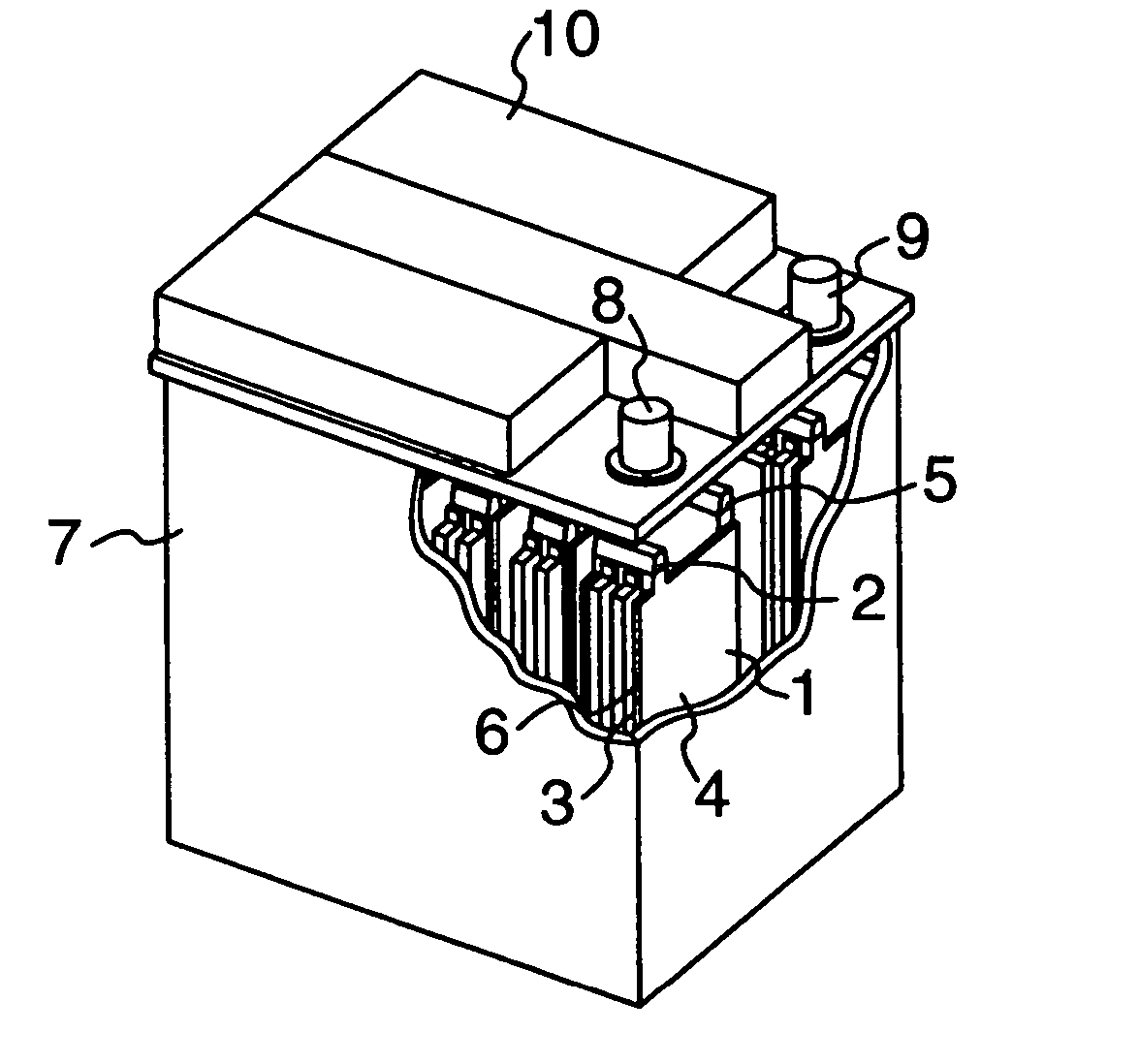
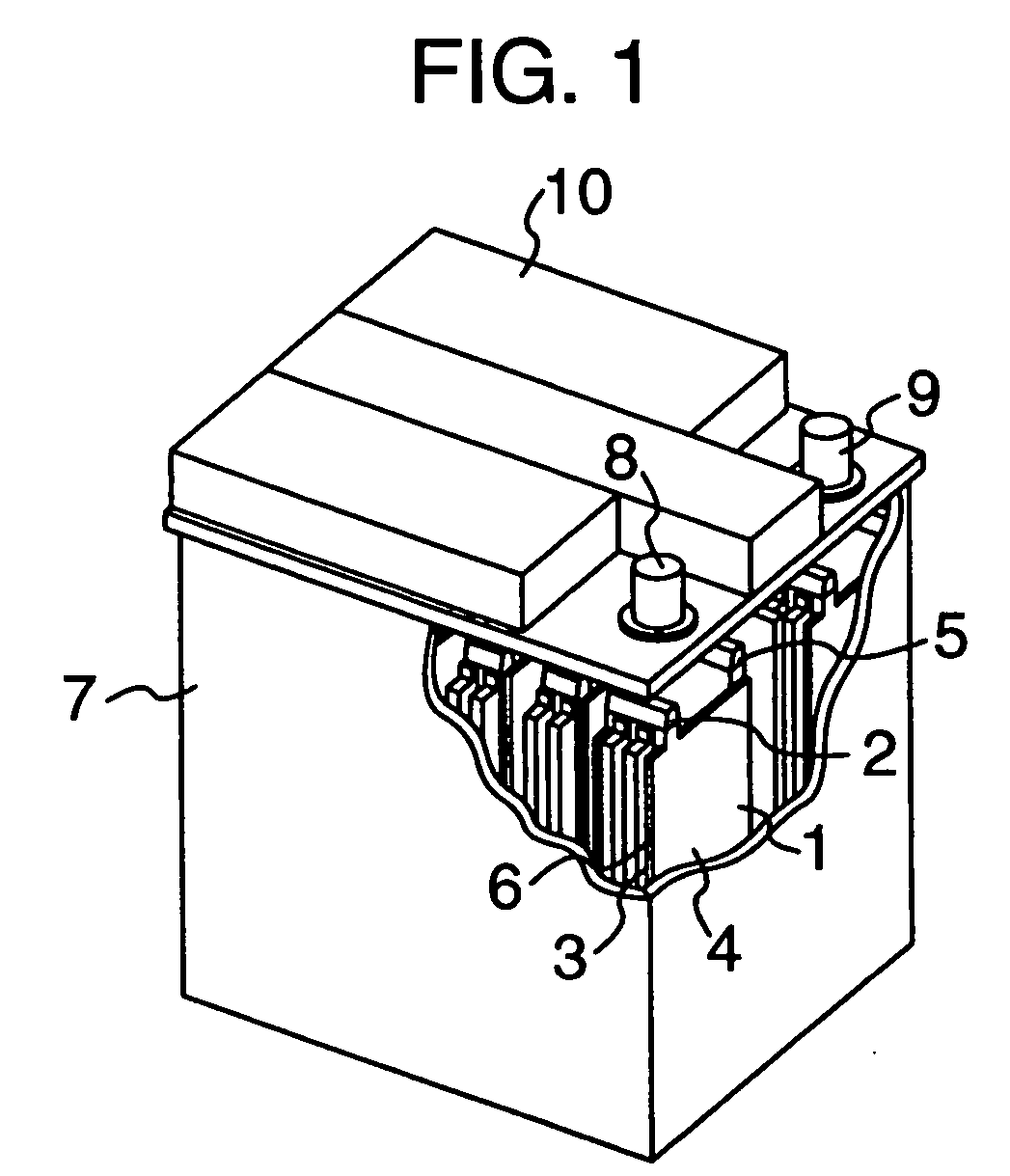
Lead-acid battery
a lead-acid battery and battery technology, applied in the field of lead-acid batteries, can solve the problems of inability to improve the solubility of lead sulfate into lead ion, and the inability to improve the solubility of lead sulfate into lead ion, and achieve the effect of improving high-efficiency charging characteristics and high-efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of Simple Substance- and / or Compound-Loaded Carbons
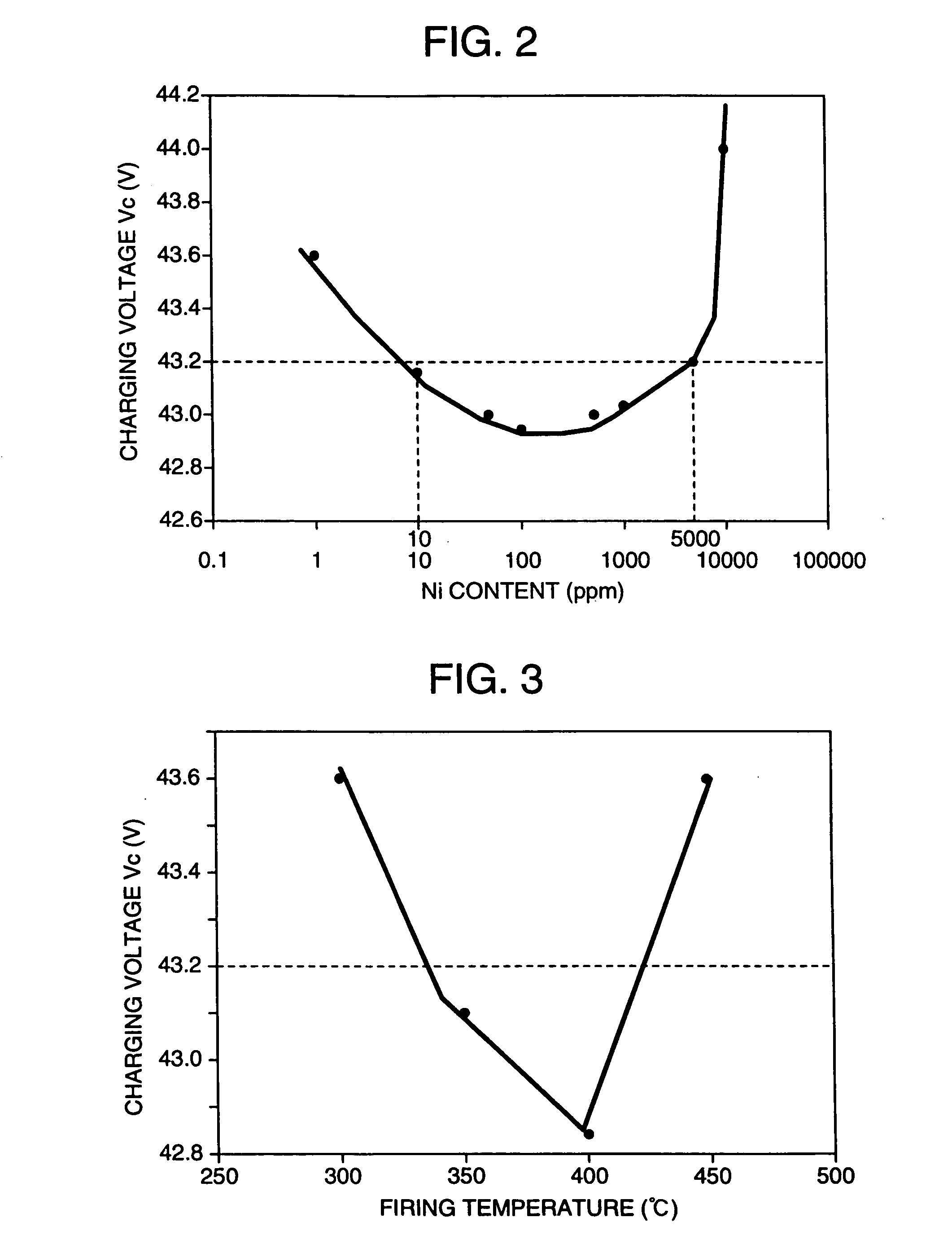
[0046] In production of simple substance- and / or compound-loaded carbons, first, aqueous nickel nitrate solutions of different concentrations were prepared. Thereto were added 10 g of acetylene black as a carbon powder and 0.1 g of a surfactant. Each resulting mixture was stirred in a water bath of 40.degree. C. Thereto was dropwise added sodium hydroxide until the pH of each mixture became 7. Then, filtration was made. The separated precipitate was washed with distilled water, dried at 120.degree. C. for 2 hours, and fired in the air, nitrogen or hydrogen at 300 to 500.degree. C. for 30 minutes to produce various nickel-loaded carbons. XRD (X-ray diffractometry) indicated that NiO was formed by the firing in the air, Ni was formed by the firing in hydrogen, and a mixture of NiO and Ni was formed by the firing in nitrogen. Incidentally, X-ray diffractometry is a method which measures the intensity of diffraction line whil...
example 2
[0058] Using, as a carbon powder, various carbons shown in Table 2, nickel-loaded carbons were produced in the same manner as in Example 1.
[0059] Lead-acid batteries were produced in the same manner as in Example 1 and measured for high-efficiency charging characteristic. Their charging voltages Vc are shown in Table 2. With all the carbons, the charging voltages Vc were below 45 V and good high-efficiency charging characteristics were obtained. Also, with mixed carbon systems thereof, the charging voltages Vc were below 45 V and good high-efficiency charging characteristics were obtained.
2TABLE 2 Amount of Primary Specific dibutyl Loaded particle surface phthalate Apparent Charging Ni diameter area absorbed density voltage amout Kind of carbon (nm) (m.sup.2 / g) (cm.sup.3 / 100 g) (g / dm.sup.3) Vc(V) (ppm) Carbon black 30 1270 495 115 44.5 10000 Ditto 11 362 270 109 44.8 15000 Ditto 30 254 174 270 43 750 Ditto 15 1475 330 152 43.1 1000 Ditto 13 560 91 400 43.7 1500 Ditto 20 140 117 310 ...
example 3
[0061] Various active carbons were used as a carbon. The contents of Cu, Ni, Mn, Al, Si, K and Mg in the active carbons were measured by ICP spectrometry and are shown in Table 4. Using these active carbons containing various amounts of impurities, lead-acid batteries were produced in the same manner as in Example 1, and their high-efficiency charging characteristics were evaluated. Their charging voltages Vc are shown in Table 4. All the charging voltages Vc were lower than 45 V and their high-efficiency charging characteristics were good.
4TABLE 4 Charging voltage Cu Ni Mn Al Si K Mg Vc Symbol (ppm) (ppm) (ppm) (ppm) (ppm) (ppm) (ppm) (V) 4-a 5 50 10 <1 <1 90 <1 43.1 4-b <1 2200 <1 10 <1 4800 <1 43.1 4-c 500 1050 850 <1 1400 <1 <1 43 4-d 55 75 75 <1 <1 105 250 43 4-e <1 <1 <1 360 <1 <1 <1 44.8 4-f <1 <1 <1 <1 <1 <1 150 44.1
PUM
| Property | Measurement | Unit |
|---|---|---|
| average primary particle diameter | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com