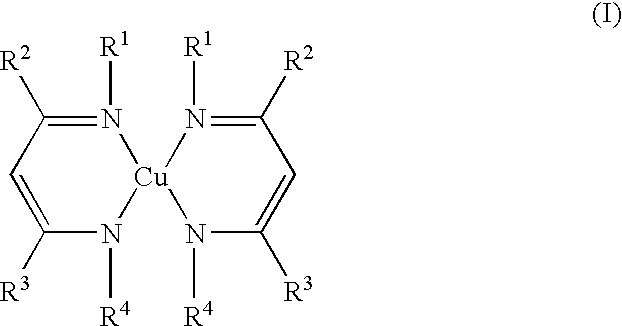
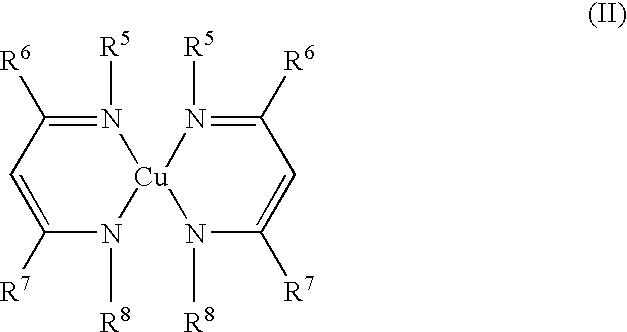
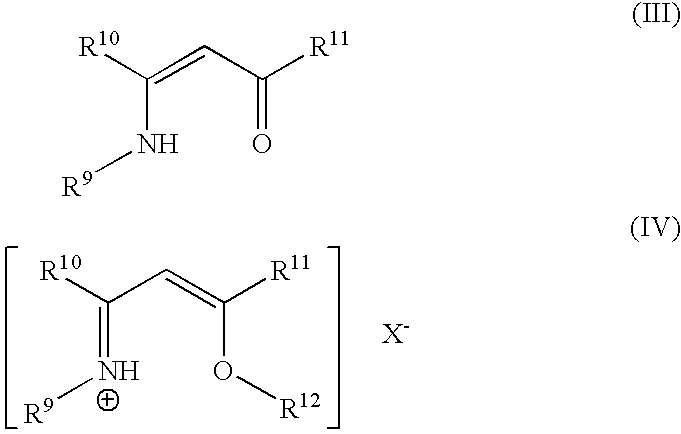
Volatile copper(II) complexes for deposition of copper films by atomic layer deposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation and Reduction of Bis(N-ethyl-4-ethylimino-2-pentene-2-aminato)copper(II)
The 1,3-diimine ligand, CH3CH2N═C(CH3)—CH2—C(CH3)═N—CH2CH3.HBF4, was prepared according to a literature procedure (McGeachin). The Cu(II)complex was prepared by reaction of the free base with copper(II) methoxide in methanol. Copper methoxide (0.268 g) was weighed into a 50-mL Erlenmeyer flask. A magnetic stir bar and 5 mL methanol were added. The free ligand was prepared from the tetrafluoroborate salt (1.00 g) by reaction with sodium methoxide; the methoxide solution was prepared by adding NaH (0.105 g) slowly to 5 mL methanol. This methanol solution was added all at once to the rapidly stirred copper methoxide solution along with an additional 5 mL methanol. A purple solution formed immediately. The mixture was stirred for 1 hour at room temperature. Solvent was removed under vacuum. The resulting solid was mixed with hexane; the resulting mixture was filtered through a sintered glass frit with ...
example 2
Preparation of MeC(NHMe)=CHC(═O)Me
Aqueous methylamine (100 g, 40% in water) was added, drop-wise, to 100 g 2,4-pentanedione. The addition was mildly exothermic; the addition rate was adjusted to keep the temperature between about 35 and 40° C. After the addition was complete, the resulting yellow liquid was stirred 1 hr at room temperature, then subjected to vacuum distillation. The still pot was heated under partial vacuum, such that the first distillation cut (presumably water) came over at 30-35° C. After this fraction was removed, the distillation was discontinued. The contents of the pot solidified upon cooling. By NMR analysis, the title compound was obtained (>95% purity) and in good yield (>90%).
example 3
Preparation of [MeC(NHMe)=CHC(OMe)Me][MeOSO3]
In the drybox, 4-(methylamino)-3-pentene-2-one (1.00 g) from Example 2 was dissolved in CH2Cl2 (2 mL) and was mixed with dimethylsulfate (1.00 g). The mixture initially formed a yellow solution, cool to the touch, but over the course of an hour, made a thick slurry with some warming. The slurry was filtered and the solids rinsed with CH2Cl2, with an isolated yield of 0.90 g (47%, based on dimethylsulfate). The NMR of the solid product was consistent with title compound.
In the drybox, 4-(methylamino)-3-pentene-2-one (1.08 g) was mixed with CH2Cl2 (0.5 mL) and the slurry combined with dimethylsulfate (1.00 g). The resulting solution was initially cool to the touch but over the course of an hour it solidified, becoming warm to the touch. This mixture was allowed to stand overnight on the stir plate at ambient temperature, then used for the subsequent reaction below. The in situ yield is nearly quantitative based on NMR analysis.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| volatility | aaaaa | aaaaa |
| stable | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com