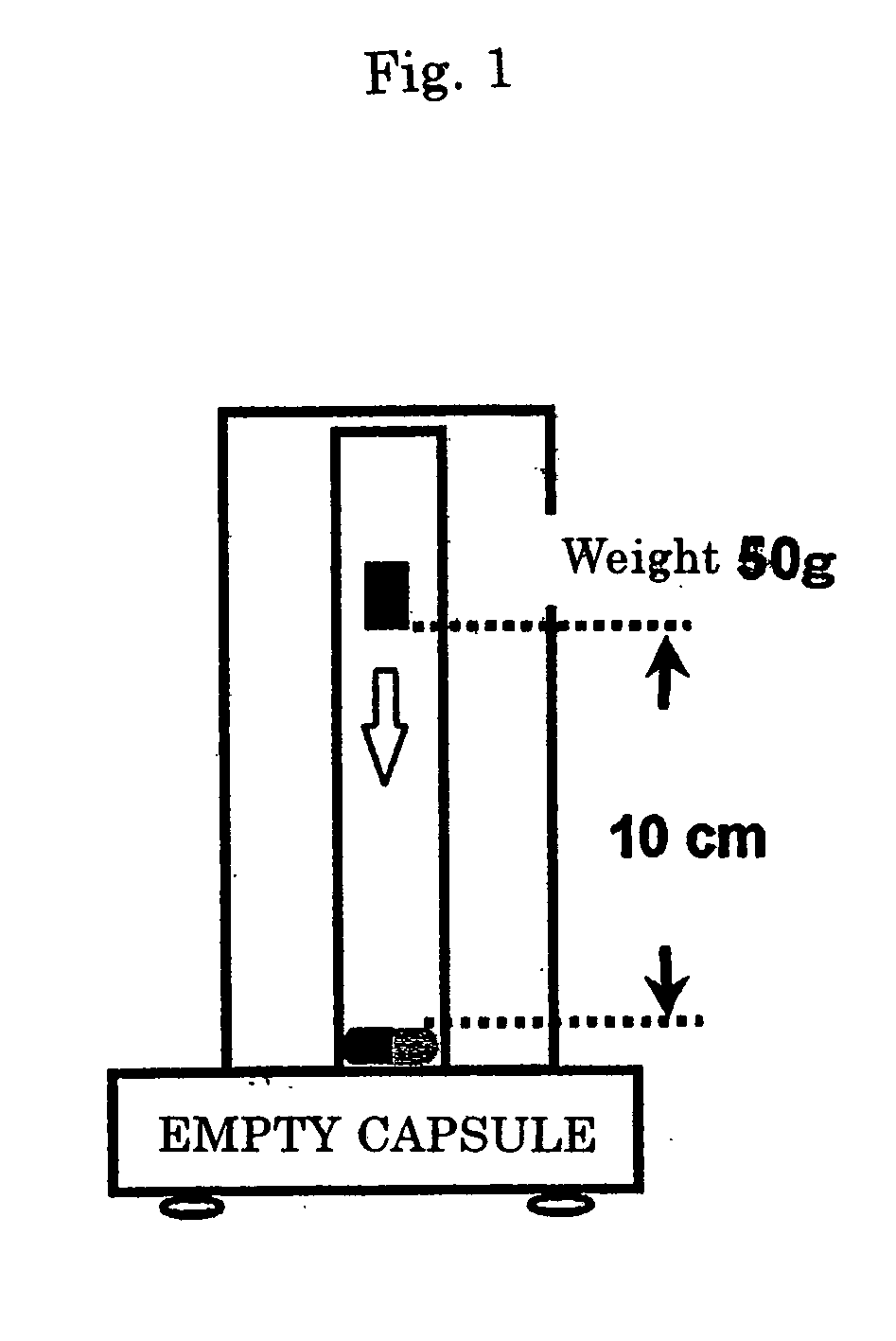
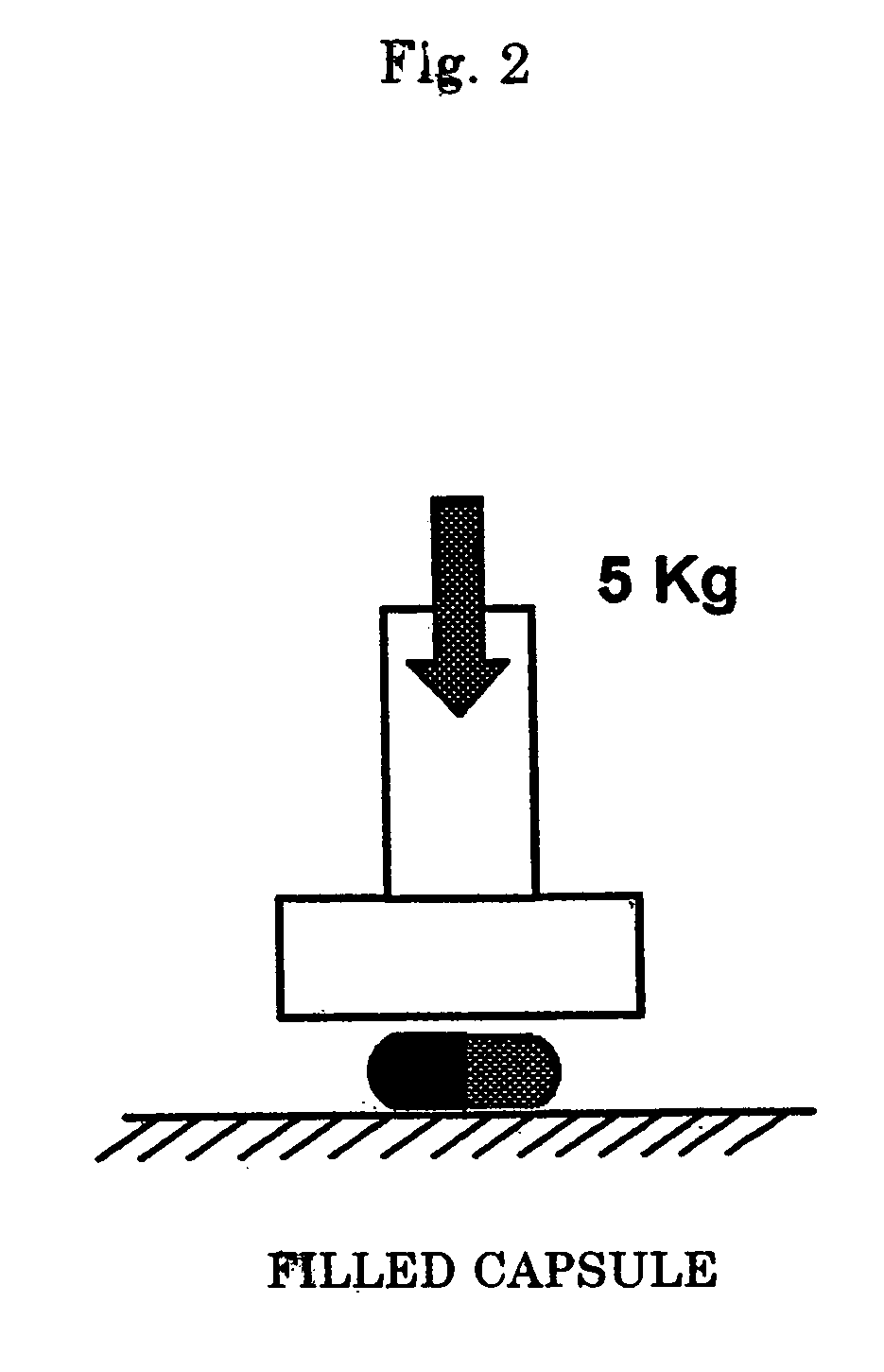
Hard capsule
a hard capsule and capsule technology, applied in the field of hard capsules, can solve the problems of poor solubility, low absorbability of alimentary canal, prone to drop or fluctuation, etc., and achieve the effect of excellent stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthesis example 1
[0055] 75 parts by weight of PVA-SH (degree of polymerization 500, degree of hydrolysis 88%, made by Kuraray Co., Ltd.) was completely dissolved in 237 parts by weight of ion-exchange water at 95° C. Next, the amounts of methacrylic acid and methyl methacrylate shown in Table 1 below were added, and after purging with nitrogen gas 3 parts by weight of t-butyl hydroperoxide was added and reaction was carried out, thus producing compounds E-1001, E-1002, E-1003 and E-1004. An aqueous solution of 15 to 20% of each of the components was prepared, and approximately 0.1 mm films were produced using a casting method. The solubilities (water solubility, solubility at pH 1.2, solubility at pH 6.8, solubility in PEG 400) and strengths (bending angle (RH 65%, dry state)) of the films produced are shown in Table 1.
[0056] In the water solubility tests, a piece of film of size 20 mm square was immersed in 10 ml of water, gentle shaking was carried out, and it was ascertained whether or not the f...
synthesis example 2
[0058] 75 parts by weight of PVA-SH (degree of polymerization 500 and 1500 mixed together, both degree of hydrolysis 88%, made by Kuraray Co., Ltd.) was completely dissolved in 237 parts by weight of ion-exchange water at 95° C. Next, the amounts of acrylic acid and methyl methacrylate shown in Table 2 below were added, and after nitrogen substitution 3 parts by weight of t-butyl hydroperoxide was added and reaction was carried out, thus producing compounds E-2001, E-2002, E-2003, E-2004, E-2005 and E-2006. The mixing proportions of the PVA-SH of degree of polymerization 500 and the PVA-SH of degree of polymerization 1500 were 50:50 (E-2001), 50:50 (E-2002), 45:55 (E-2003), 40:60 (E-2004), 20:80 (E-2005) and 10:90 (E-2006). An aqueous solution of 15 to 20% of each of the components was prepared, and approximately 0.1 mm films were produced using a casting method. The solubilities and strengths of the films produced were measured as in Synthesis Example 1, and are shown in Table 2. ...
synthesis example 3
[0059] 75 parts by weight of PVA-SH (degree of polymerization 500 and 1500 mixed together in a ratio of 1:9, both degree of hydrolysis 88%, made by Kuraray Co., Ltd.) was completely dissolved in 237 parts by weight of ion-exchange water at 95° C. The amounts of methacrylic acid and methyl methacrylate shown in Table 3 below were then added thereto, and after nitrogen substitution 3 parts by weight of t-butyl hydroperoxide was added and reaction was carried out, thus producing compounds E-3001, E-3002, E-3003. An aqueous solution of 15 to 20% of each of the components was prepared, and approximately 0.1 mm films were produced using a casting method. The solubilities and strengths of the films produced were measured as in Synthesis Example 1, and are shown in Table 3.
TABLE 3Polymer composition ratios and film propertyvalues for Synthesis Example 3E-3001E-3002E-3003CompositionPVA-SH757575MAA7.5105MMA17.51520Film solubilityWater solubilityYesYesYespH 1.2 solubilityYesYesYespH 6.8 solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com