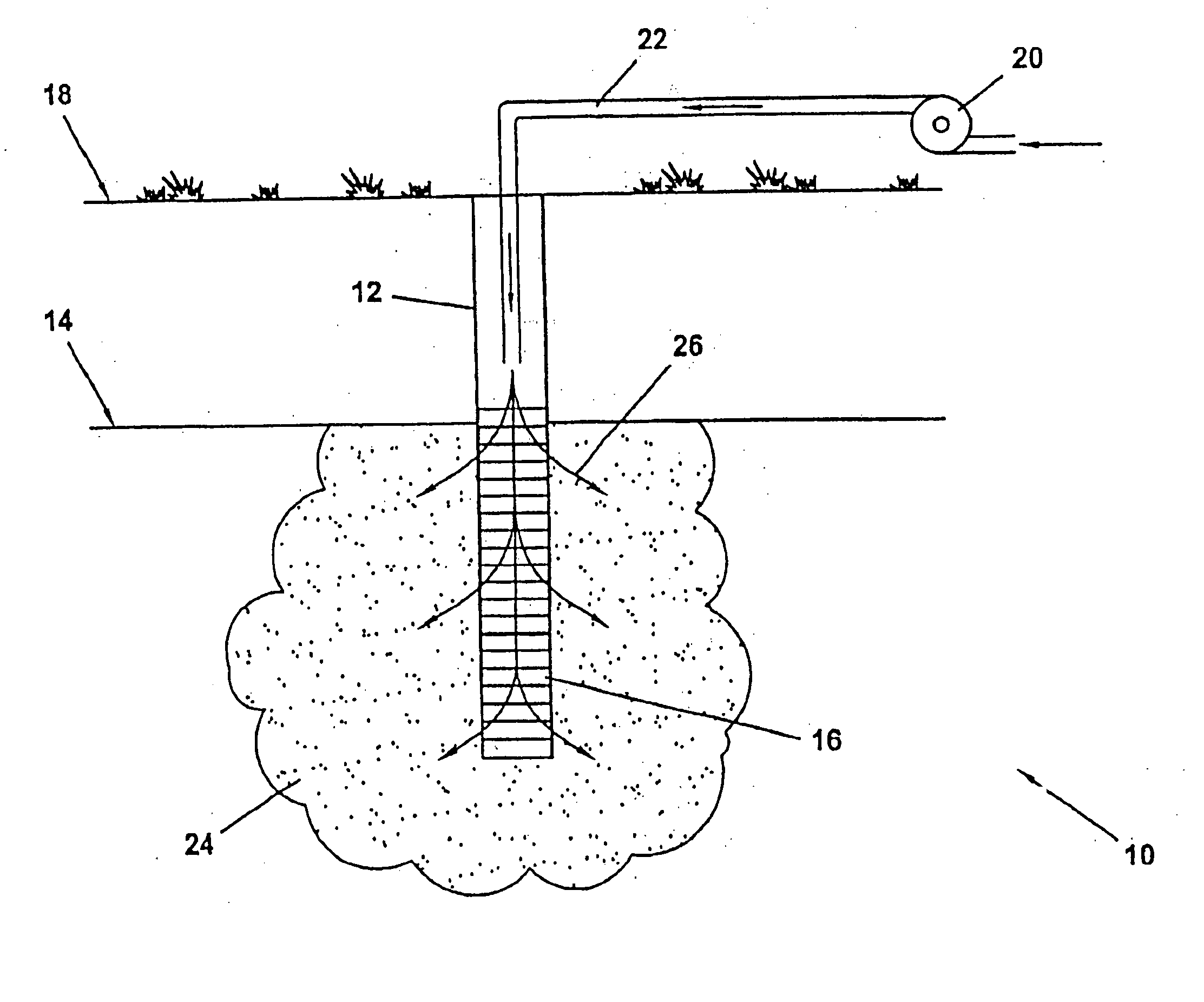
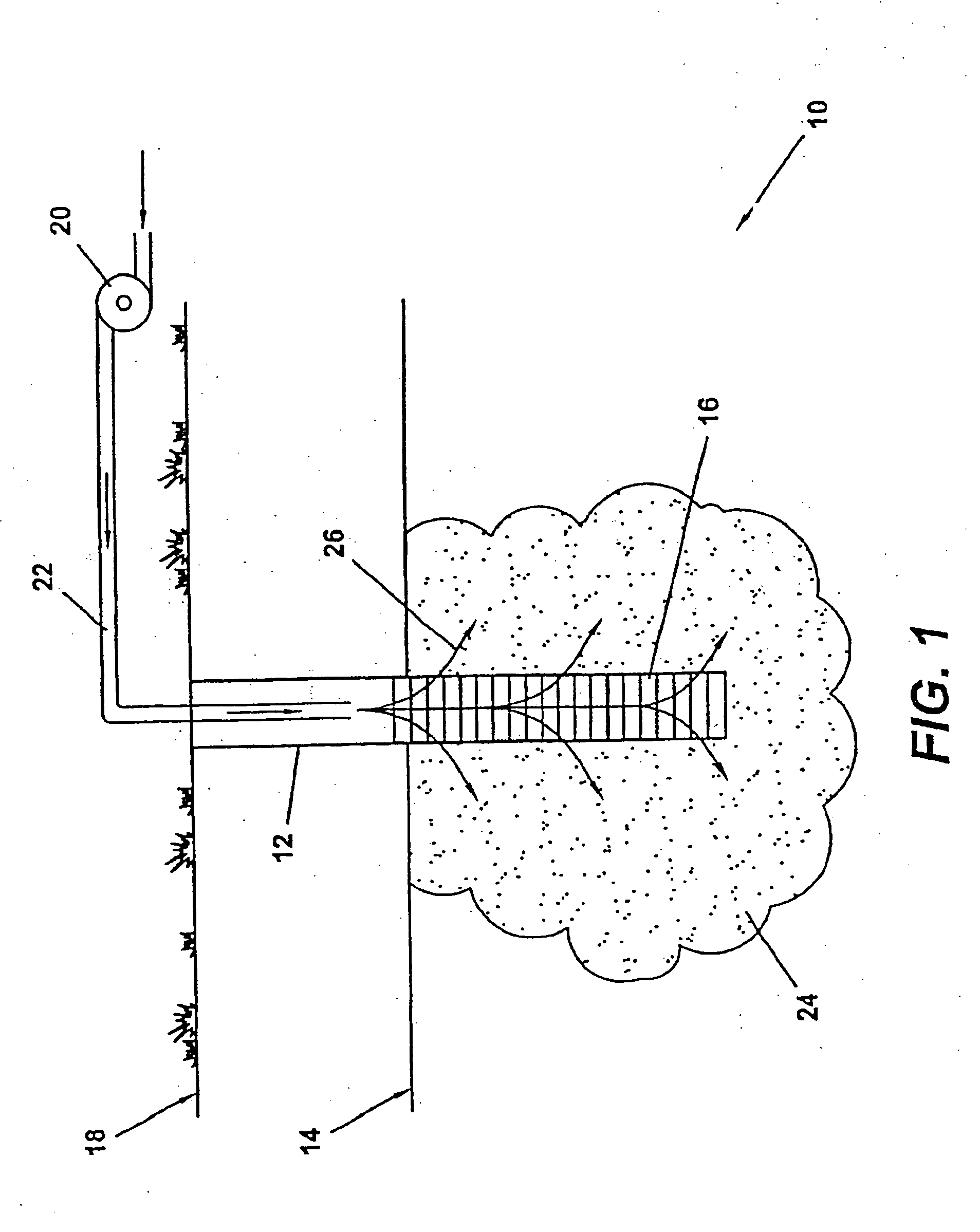
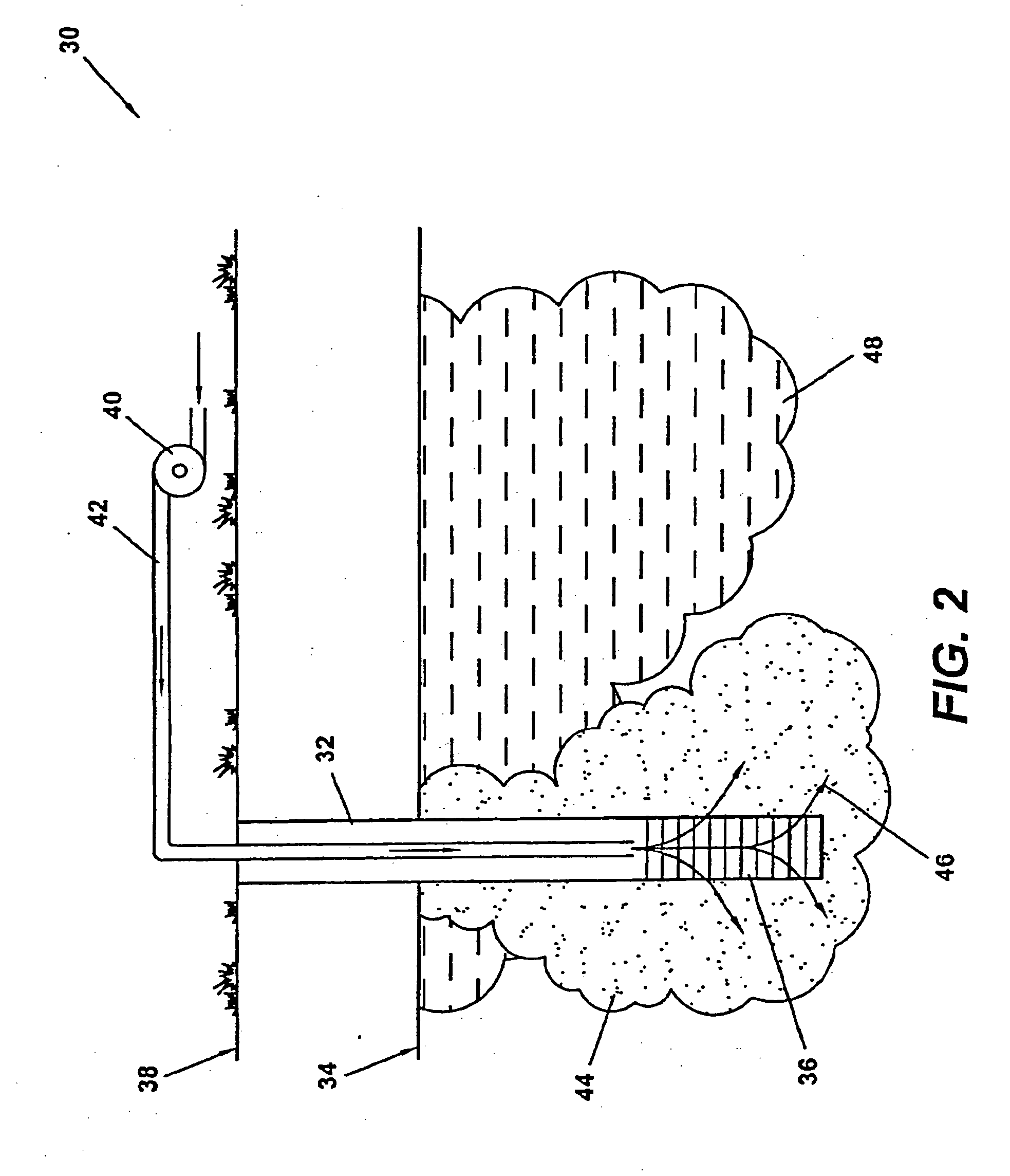
Method of making and using nanoscale metal
a nano-scale metal and metal technology, applied in the field of making and using nano-scale metals, can solve the problems of inability to meet the requirements of large-scale application in groundwater remediation, inability to achieve full-scale production in ton quantities, and inability to meet the requirements of large-scale use, etc., to achieve greater reaction kinetics, control of the transport properties of colloids, and the effect of increasing surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0023] The mechanical agitation processes for grinding metal particles can be divided into two types; those that rely on squashing the metal particles between rotating plates or elements, wherein one or both plates may be rotating; and those that rely on impact to fracture larger metal particles into smaller particles. Particle feed rates, grinding time, unit capacity and the physical / chemical properties of the metal to be ground must all be considered in deciding which method should be used for grinding and, more importantly, which method can be used to produce nanoscale colloids in commercial quantities at a cost that is viable for the use of the material in high quantities such as tons.
[0024] Rotary plate grinders are limited in their instantaneous capacity, the type of metals which can be attrited in them, and the duration of grinding that is required to produce the desired size range. In general the clearances associated with rotary plate grinders do not allow for the large sc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com