Photosensitive polymer and chemically amplified photoresist composition including the same
a photoresist composition and polymer technology, applied in the field of photosensitive polymer and chemically amplified photoresist composition, can solve the problems of steep patterns that cannot be formed by the conventional g-line(436 nm) or i-line(365 nm) exposure process, and are not stabl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of poly(HS-co-DEES-co-Styrene-co-CBCPS)
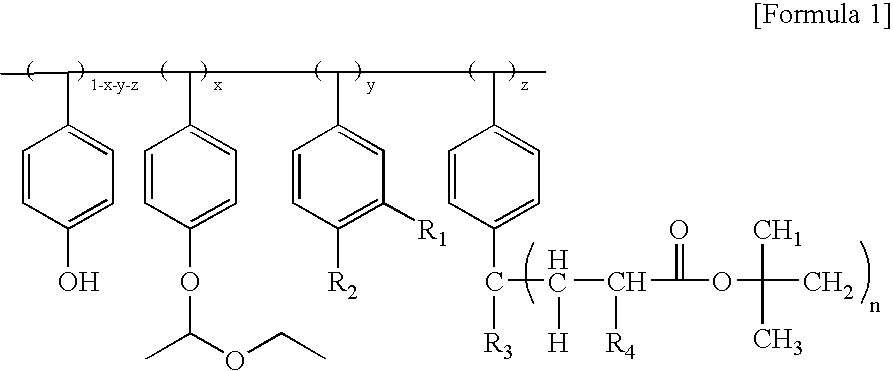
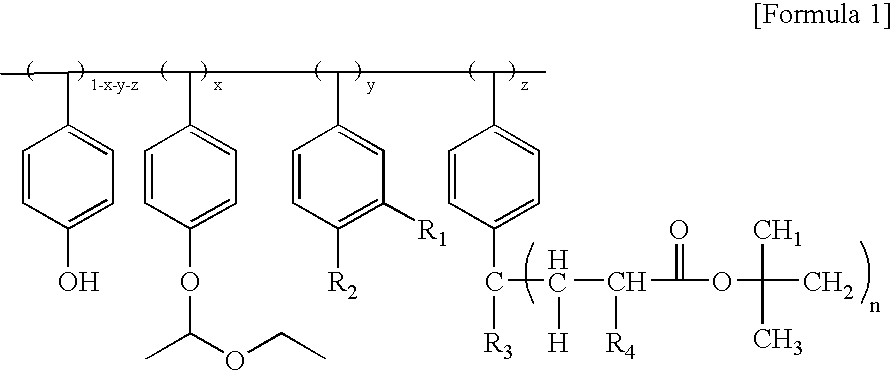
(Formula 1a)
[0024] a) Synthesis of 4-Cyanomethylstyrene(CyMS)
[0025] As shown in the following Reaction 1a, 49.01 g of sodium cyanide (NaCN), 70.07 g of water and 50.96 g of ethanol were added into a 500 ml 4-neck flask equipped with a mechanical stir, and the temperature of the solution was elevated to 60° C. to completely dissolve NaCN. To the solution, 87.50 g of 4-chloromethylstyrene was slowly added and the reaction was carried out for 3 hours at the temperature of 60-70° C. After completion of the reaction, the solution was cooled to 40° C., 100 g of diethylether was added to the solution, and the diethylether layer was separated. The separated organic layer was extracted with 300 g of water three times. The water layer was extracted with 50 g of diethylether and the diethylether extract was added to the organic layer. The separated organic layer was dried with magnesium sulfate for one day, and then the organic solvent was r...
example 2
Synthesis of poly(HS-co-pEES-co-pTBS-co-CBCPS)
(Formula 1b)
[0029] As shown in the following Reaction 2, except for using 17.63 g of para-tert-butoxy styrene instead of 10.42 g of styrene as the monomer, using 2.27 g of AIBN as the initiator and using 11.89 ml of 28 weight % NH3 aqueous solution, the same method as described in Example 1 was carried out to obtain 47.73 g of poly(HS-co-pEES-co-pTBS-co-CBCPS) of Formula 1b.
[0030] In Reaction 2,1-x-y-z, x, y and z are the same as defined in Formula 1.
example 3
Synthesis of poly(HS-co-DEES-co-tBocS-co-CBCPS)
(Formula 1c)
[0031] a) Synthesis of Poly(HS-co-pEES-co-CBCPS)
[0032] As shown in the following Reaction 3a, 200 ml of tetrahydrofuran(THF) was introduced into a 500 ml 4-neck flask equipped with a refluxing cooler, a temperature controlling apparatus and a nitrogen gas introducing apparatus, and was stirred for 30 minutes with introducing nitrogen. To the reactor, 58.39 g of 4-acetoxystylene, 53.83 g of 4-(1-ethoxy)-ethoxystylene, 15.98 g of CBCPS prepared in Example 1(b), and 2.23 g of AIBN as the initiator were added. The temperature of the reactor was elevated to 40° C. and the reactant was stirred for 30 minutes under a nitrogen atmosphere. The temperature of the reactor was then elevated to 60-70° C. and the reactant was stirred for 24 hours under reflux. After completion of the reaction, the temperature of the reactant was lowered to room temperature(25° C.) and the reactant was poured into 2 l of hexane to obtain the precipitat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| polydispersity | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com