Nuclear transfer embryo formation method
a technology of nuclear transfer and embryos, applied in the field of nuclear transfer embryo formation methods, can solve the problems of inherently invasive and clearly damaging the spatial organization of cytoplasts, inability to enucleate cytoplasts, and inability to fully enucleate cytoplasts, so as to improve the yield of competent embryos
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Improved Method of Cloning Through the Introduction of Donor Cell Nucleus Prior to Completion of Enucleation Process
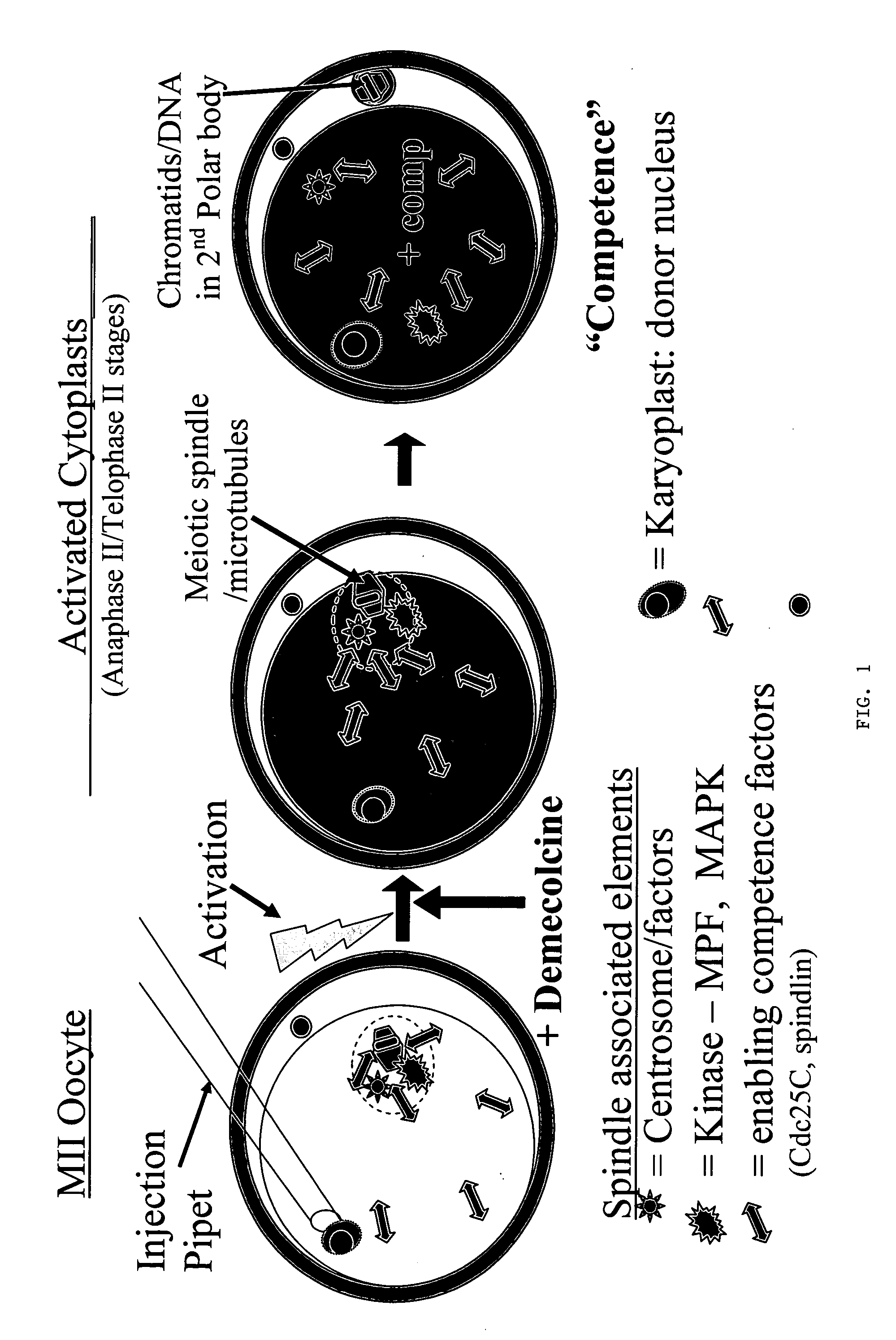
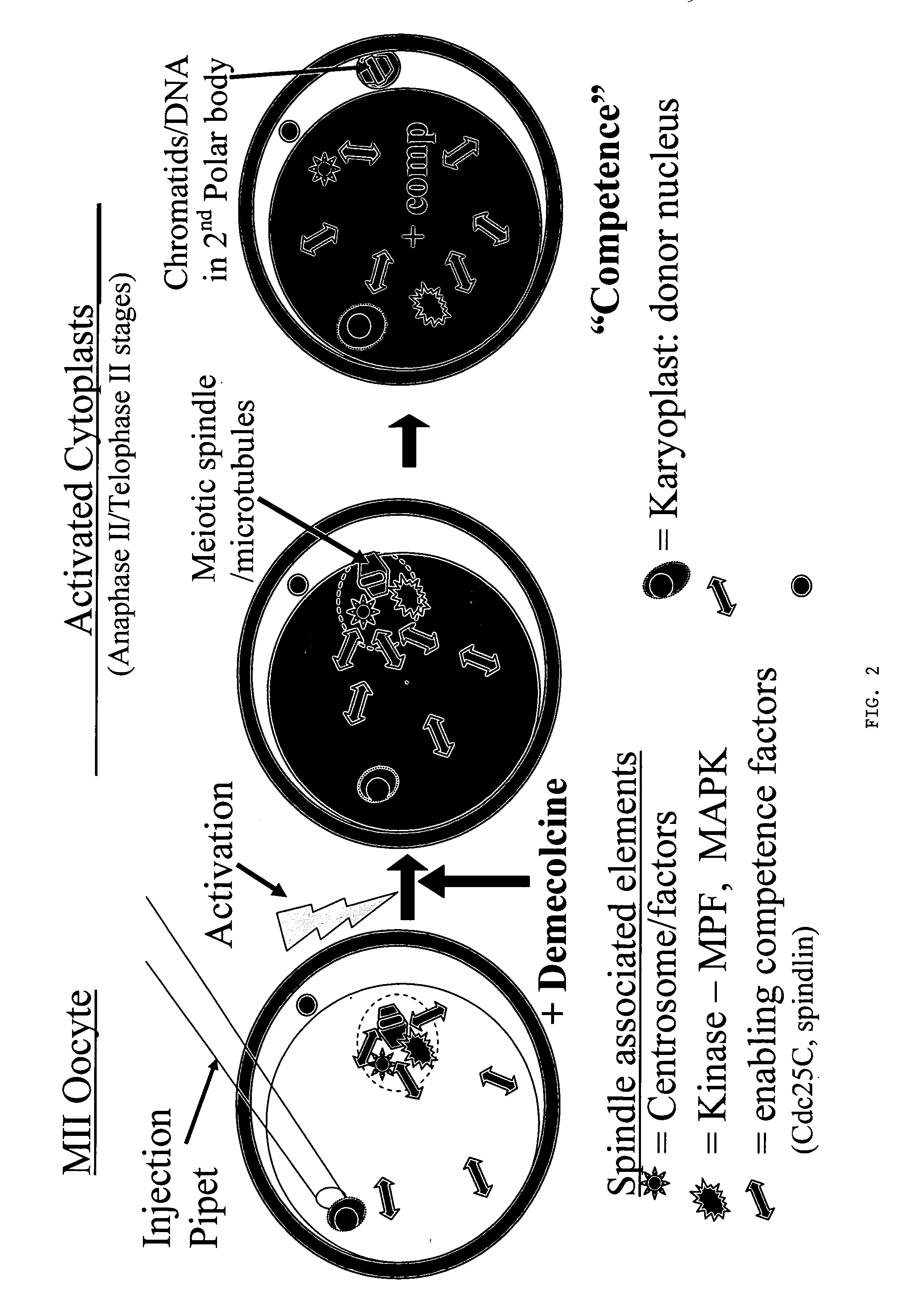
[0081] Progress has continued with experiments designed to compare in vitro development of control embryos (parthenotes) with that of reconstructed NT embryos prepared by conventional NT, telophase NT, IE and a novel simultaneous IE-NT paradigm (SIE / NT; see Table 1 below). As in all previous experiments, abattoir-sourced oocytes were obtained having been submitted to 26 h of maturation in ACM media @5% CO2 in air. Oocytes were denuded (enzyme / vortex) and activated (5 μM ionomyciin for 5 min, + / −CHX). See Example 2 for detail on methods. IE was performed by treatment of oocytes with demecolcine (0.4 μg / ml) starting at 1.5 h and ending 5 h post-activation (p.a.) and nuclei were injected 1.5-3 h p.a. In the case of SIE / NT, donor karyoplasts (fibroblasts) were injected immediately prior to or immediately after activation. In vitro embryo development was evaluated over a p...
example 2
Demecolcine-Induced Oocyte Enucleation for Somatic Cell Cloning: Coordination Between Cell Cycle Egress, Kinetics of Cortical Cytoskeletal Interactions, and Second Polar Body Extrusions
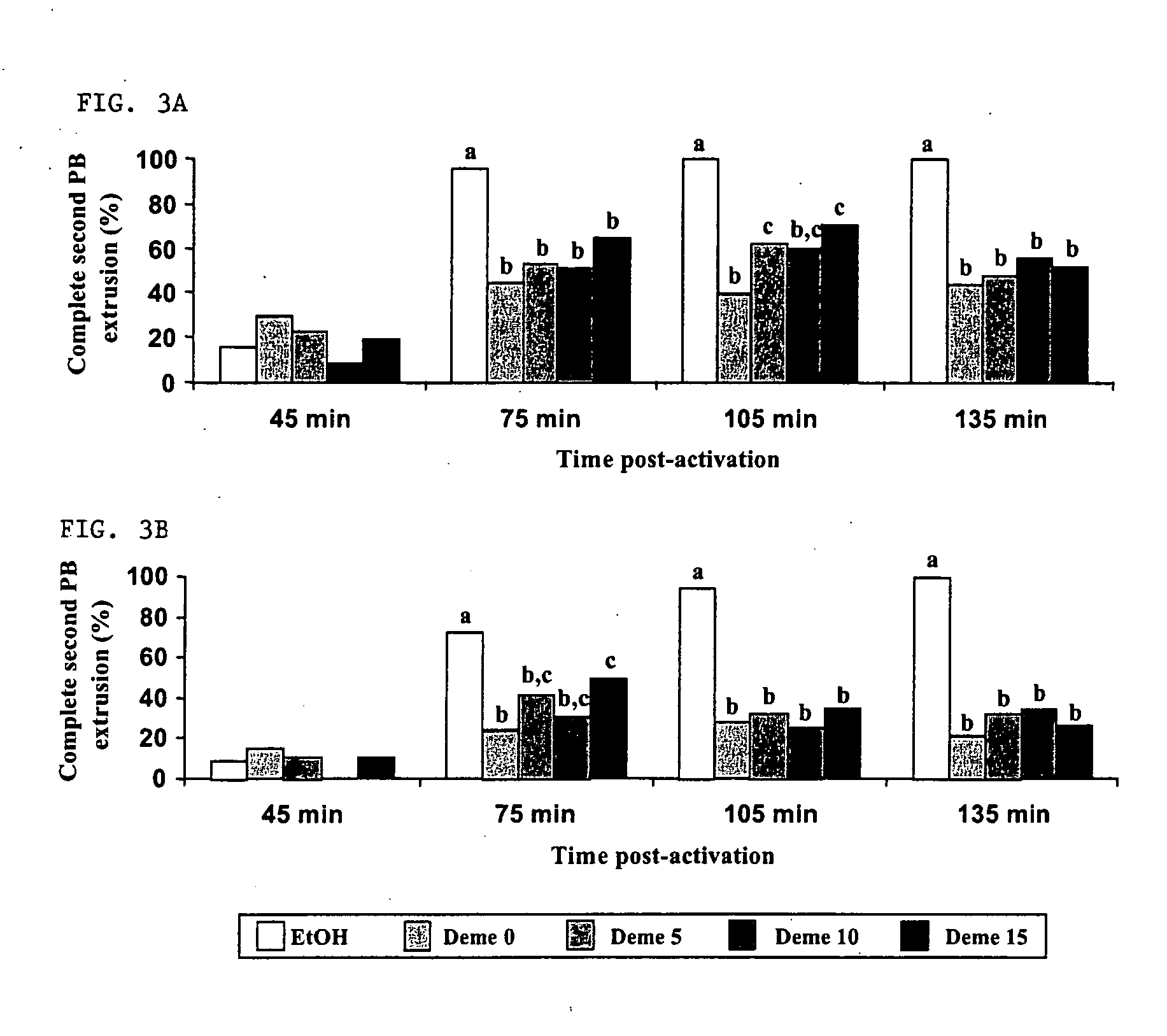
[0084] Studies were designed to further explore the use of pharmacological agents to create developmentally competent enucleated mouse oocytes for animal cloning by somatic cell nuclear transfer. Metaphase-II oocytes from CF-1 and B6D2F1 strains were activated with ethanol and subsequently exposed to demecolcine at various times post-activation. Chromosome segregation, spindle dynamics and polar body (PB) extrusion were monitored by fluorescence microscopy using DNA, microtubule and microfilament selective probes. Exposure to demecolcine did not affect rates of oocyte activation induced by ethanol but did disrupt the coordination of cytokinesis and karyokinesis, suppressing the extent and completion of spindle rotation and second PB extrusion in a strain-dependent manner. Moreover, strain and treatme...
example 3
Activated Bovine Cytoplasts Produced by Induced Enucleation Support Development of Nuclear Transfer Embryos In Vitro
[0115] Poor efficiency of somatic cell NT has been associated with the preparation of developmentally competent enucleated cytoplasts. Induced enucleation (IE) of mouse oocytes has been shown to support enhanced term development of cloned mice. This study characterized the kinetics and phenotypic progression of bovine oocytes subjected to IE, and evaluated their developmental competence to support NT embryo development in vitro. In vitro matured (26 h) oocytes were denuded, activated (5 pM ionomycin, 5 min, then 10 μg / mL cycloheximide, 5 h) and cultured for up to 5 h post-activation (pa). Oocyte enucleation was induced by demecolcine (0.4 μg / ml, DM) exposure at 30, 60, 90 and 120 min post activation for various time periods (1 to 4.5 h). Activation rates and meiotic progression of control and DM treated oocytes (n=31-49 / gp) was evaluated at 5 hpa by immunofluorescence...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time- | aaaaa | aaaaa |
| time- | aaaaa | aaaaa |
| time- | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com