ErbB antagonists for pain therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
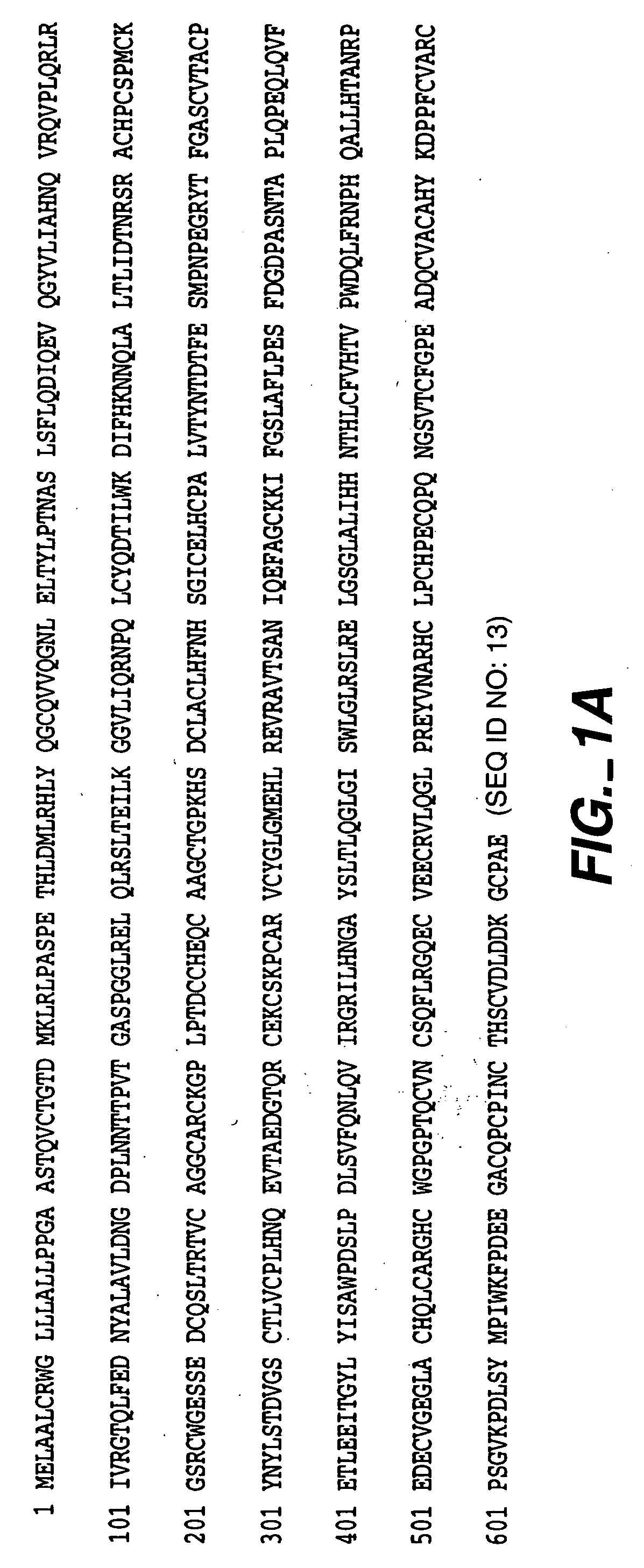
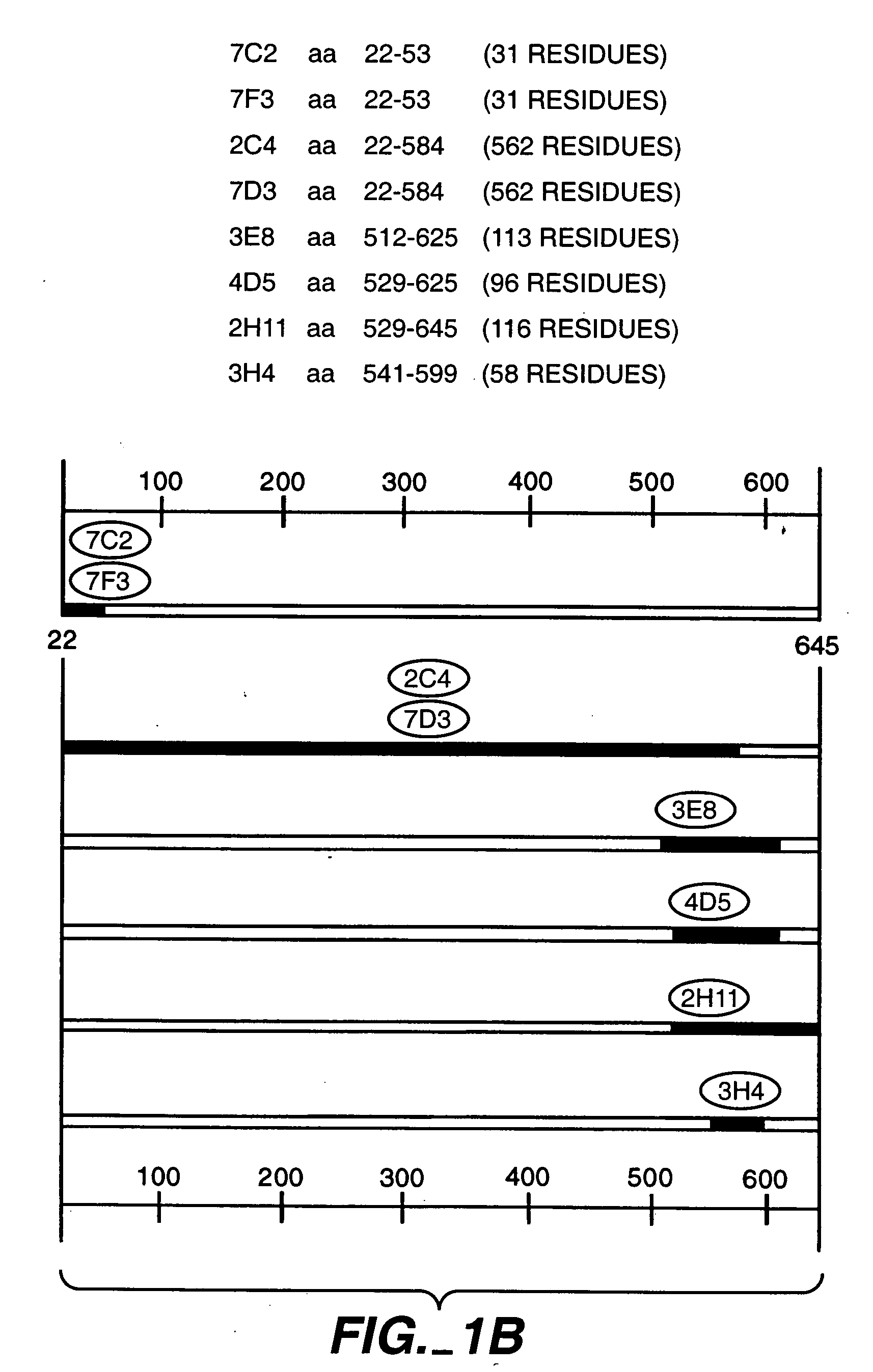
Production and Characterization of Monoclonal Antibody 2C4
[0308] The murine monoclonal antibodies 2C4, 7F3 and 4D5 which specifically bind the extracellular domain of ErbB2 were produced as described in Fendly et al., Cancer Research 50:1550-1558 (1990). Briefly, NIH 3T3 / HER2-3400 cells (expressing approximately 1×105 ErbB2 molecules / cell) produced as described in Hudziak et al Proc. Natl. Acad. Sci. (USA) 84:7158-7163 (1987) were harvested with phosphate buffered saline (PBS) containing 25 mM EDTA and used to immunize BALB / c mice. The mice were given injections i.p. of 107 cells in 0.5 ml PBS on weeks 0, 2, 5 and 7. The mice with antisera that immunoprecipitated 32P-labeled ErbB2 were given i.p. injections of a wheat germ agglutinin-Sepharose (WGA) purified ErbB2 membrane extract on weeks 9 and 13. This was followed by an i.v. injection of 0.1 ml of the ErbB2 preparation and the splenocytes were fused with mouse myeloma line X63-Ag8.653.
[0309] Hybridoma supernatants were screened...
example 2
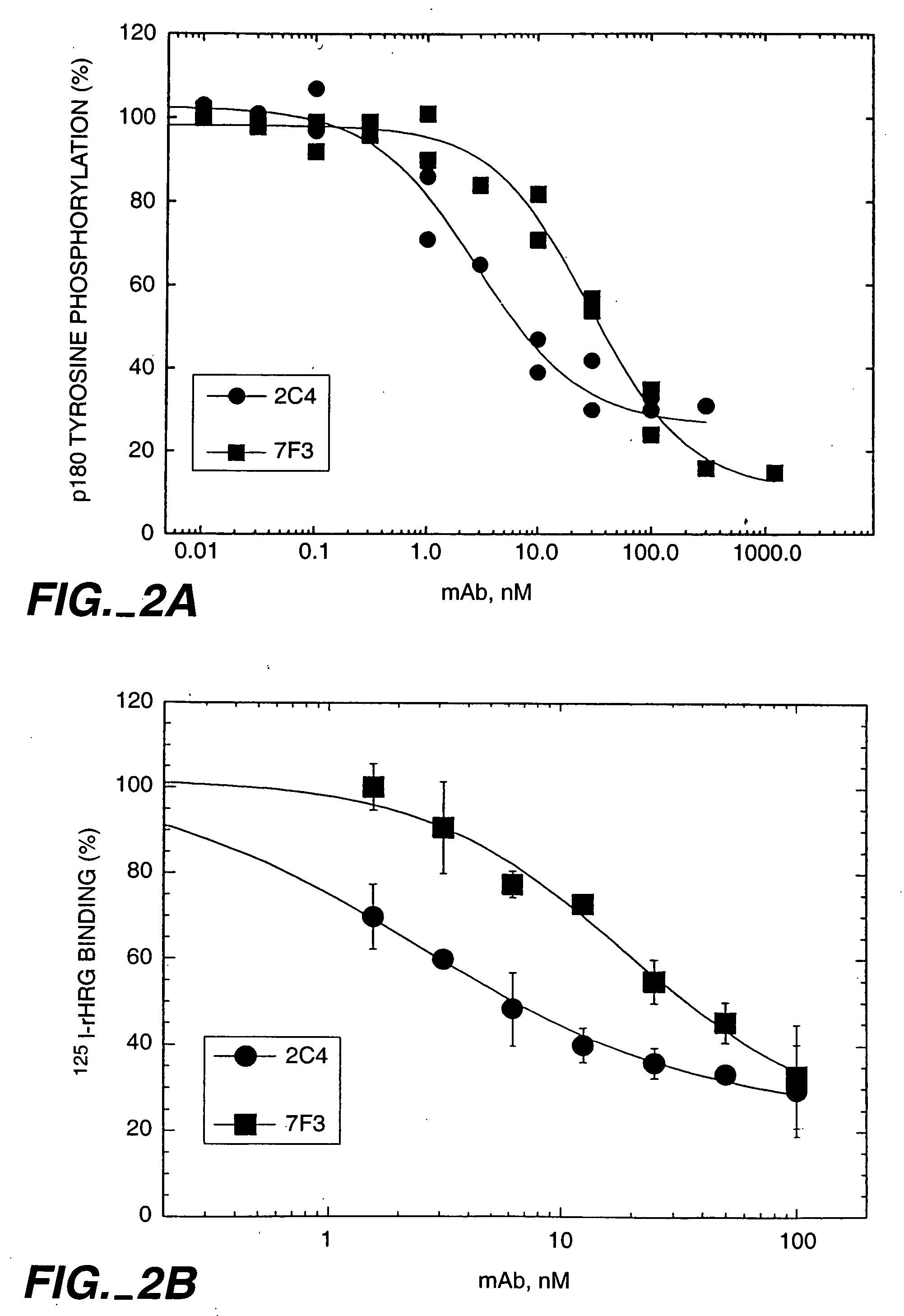
HRG Dependent Association of ErbB2 with ErbB3 is Blocked by Monoclonal Antibody 2C4
[0319] The ability of ErbB3 to associate with ErbB2 was tested in a co-immunoprecipitation experiment. 1.0×106 MCF7 or SK-BR-3 cells were seeded in six well tissue culture plates in 50:50 DMEM / Ham's F12 medium containing 10% fetal bovine serum (FBS) and 10 mM HEPES, pH 7.2 (growth medium), and allowed to attach overnight. The cells were starved for two hours in growth medium without serum prior to beginning the experiment.
[0320] The cells were washed briefly with phosphate buffered saline (PBS) and then incubated with either 100 nM of the indicated antibody diluted in 0.2% w / v bovine serum albumin (BSA), RPMI medium, with 10 mM HEPES, pH 7.2 (binding buffer), or with binding buffer alone (control). After one hour at room temperature, HRG was added to a final concentration of 5 nM to half the wells (+). A similar volume of binding buffer was added to the other wells (−). The incubation was continued ...
example 3
Humanized 2C4 Antibodies
[0325] The variable domains of murine monoclonal antibody 2C4 were first cloned into a vector which allows production of a mouse / human chimeric Fab fragment. Total RNA was isolated from the hybridoma cells using a Stratagene RNA extraction kit following manufacturer's protocols. The variable domains were amplified by RT-PCR, gel purified, and inserted into a derivative of a pUC119-based plasmid containing a human kappa constant domain and human CH1 domain as previously described (Carter et al. PNAS (USA) 89:4285 (1992); and U.S. Pat. No. 5,821,337). The resultant plasmid was transformed into E. coli strain 16C9 for expression of the Fab fragment. Growth of cultures, induction of protein expression, and purification of Fab fragment were as previously described (Werther et al. J. Immunol. 157:4986-4995 (1996); Presta et al. Cancer Research 57: 4593-4599 (1997)).
[0326] Purified chimeric 2C4 Fab fragment was compared to the murine parent antibody 2C4 with respe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com