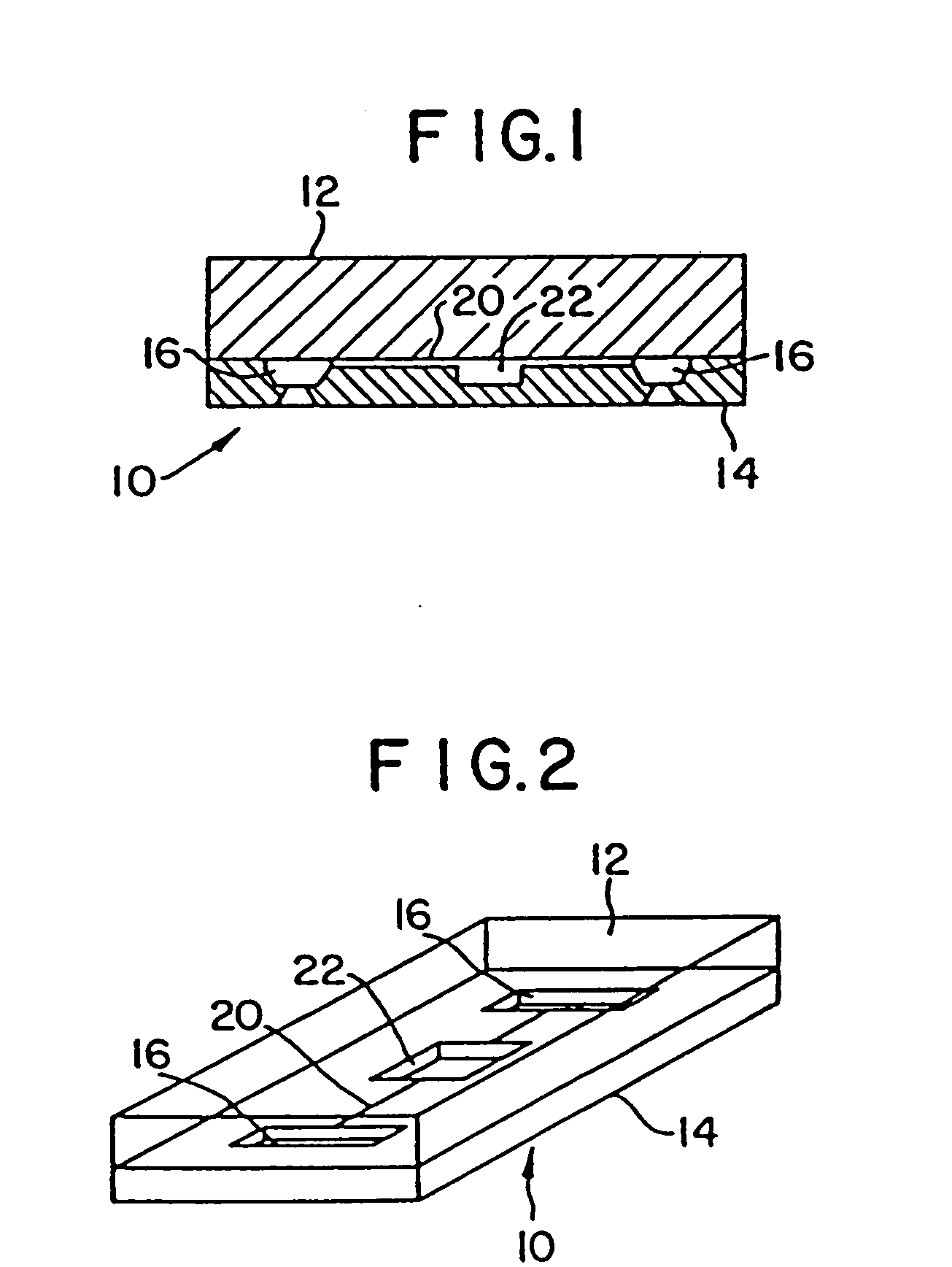
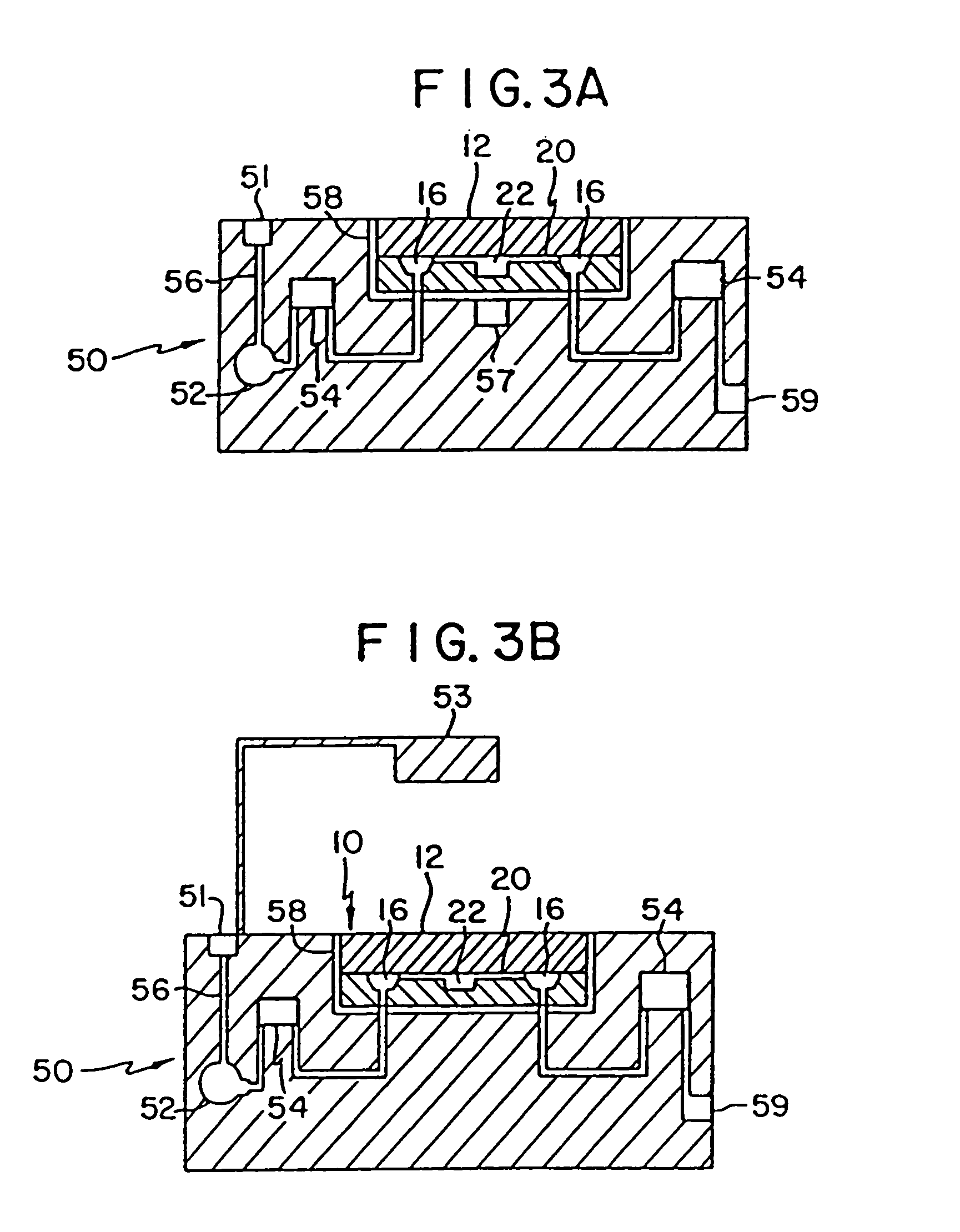
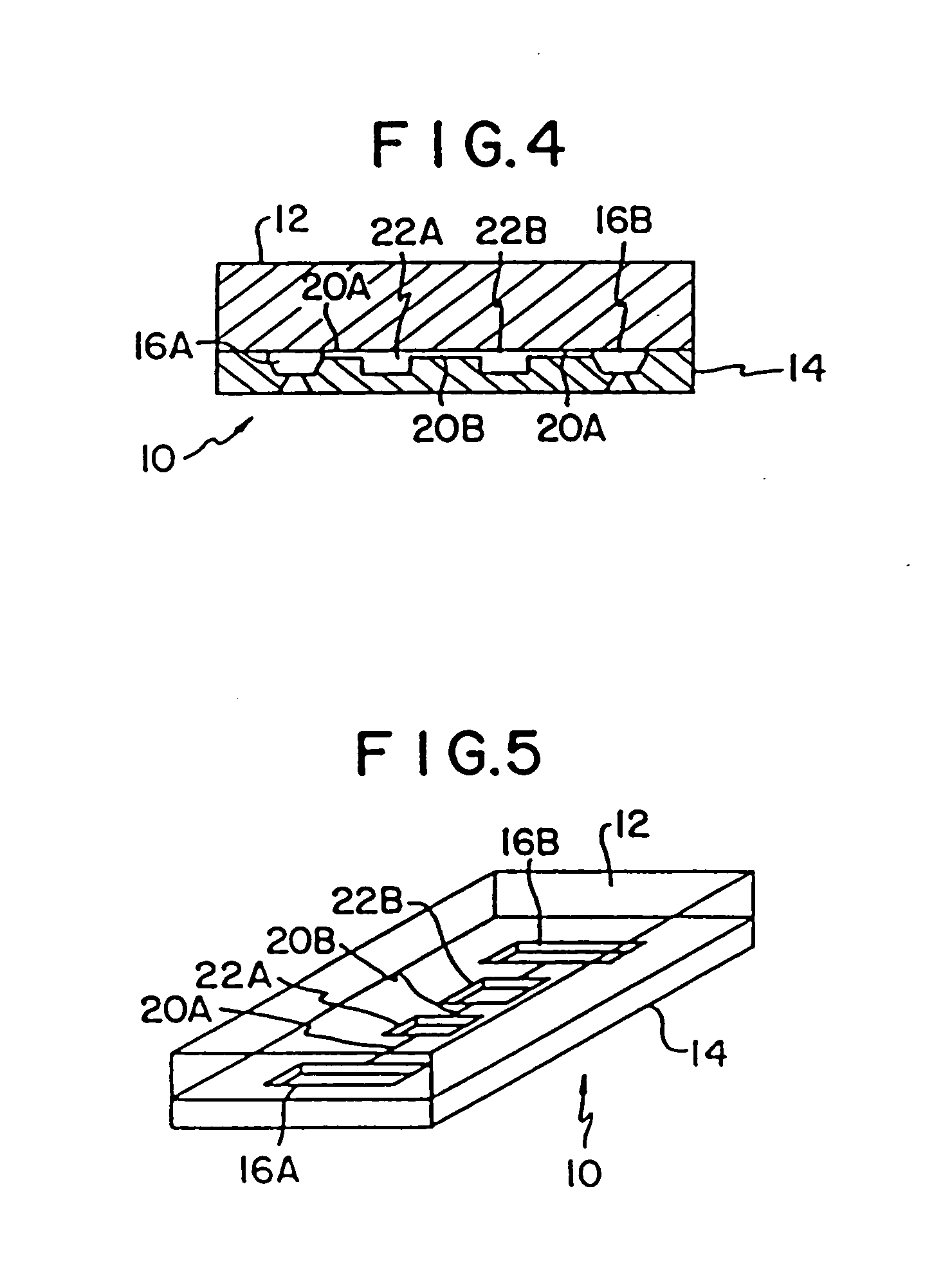
Mesoscale polynucleotide amplification analysis
a polynucleotide and mesoscale technology, applied in the field of methods and apparatus for conducting analyses, can solve the problems of not fully exploiting the potential of mesoscale structures in the life sciences, not producing data associated with an analytical device, and not fully exploring the potential of using mesoscale devices for the analysis of biological fluids. , to achieve the effect of reducing reagent costs, reducing storage and shipping costs, and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0070] A polymerase chain reaction is performed in the device illustrated schematically in FIG. 11. To perform a PCR analysis to detect a polynucleotide in a cell, a sample cell lysate is added to a buffered solution of Taq polymerase, nucleoside triphosphates, polynucleotide primers and other reagents required for PCR. The cell sample lysate is delivered via the appliance through entry port 16A to PCR reaction chamber 22A. Ports 16A and 16D are closed by means of valves included in the appliance, while port 16B and 16C are open. The microprocessor and temperature control element in the appliance are used to implement a temperature cycle in reaction chamber 22A between 94° C., for polynucleotide dehybridization, and 65° C., for polymerase reaction. After the polymerase chain reaction is complete, port 16C is closed, 16D opened, and the pump in the appliance connected to port 16B used to deliver the sample from. the PCR reaction. chamber 22A through flow channel 20B to the detection ...
example 2
[0071]FIG. 12 depicts schematically a device 10 including substrate 14 used to separate a nucleic acid from a subpopulation of cells in a mixture in a biological fluid sample, and then to perform an assay for a particular nucleotide sequence. Microfabricated on device 10 is a mesoscale flow path 20 which includes a cell separation chamber 22A, a cell lysis chamber 22B, a filter region 24, a PCR reaction chamber comprising sections 22C and 22D, and a fractal detection region 40. The mesoscale flow system 20 is also provided with fluid entry / exit ports 16A, 16B, 16C and 16D. The device is used in combination with an appliance, such as appliance 50, shown in FIG. 6A.
[0072] Initially, the valves in the appliance are used to close ports 16C and 16D, while ports 16A and 16B are open. A sample containing a mixture of cells is. directed to the sample inlet port 16A by the pump 52 in the appliance, and flows through the mesoscale flow path 20 to separation chamber 22A. Chamber 22A contains ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com