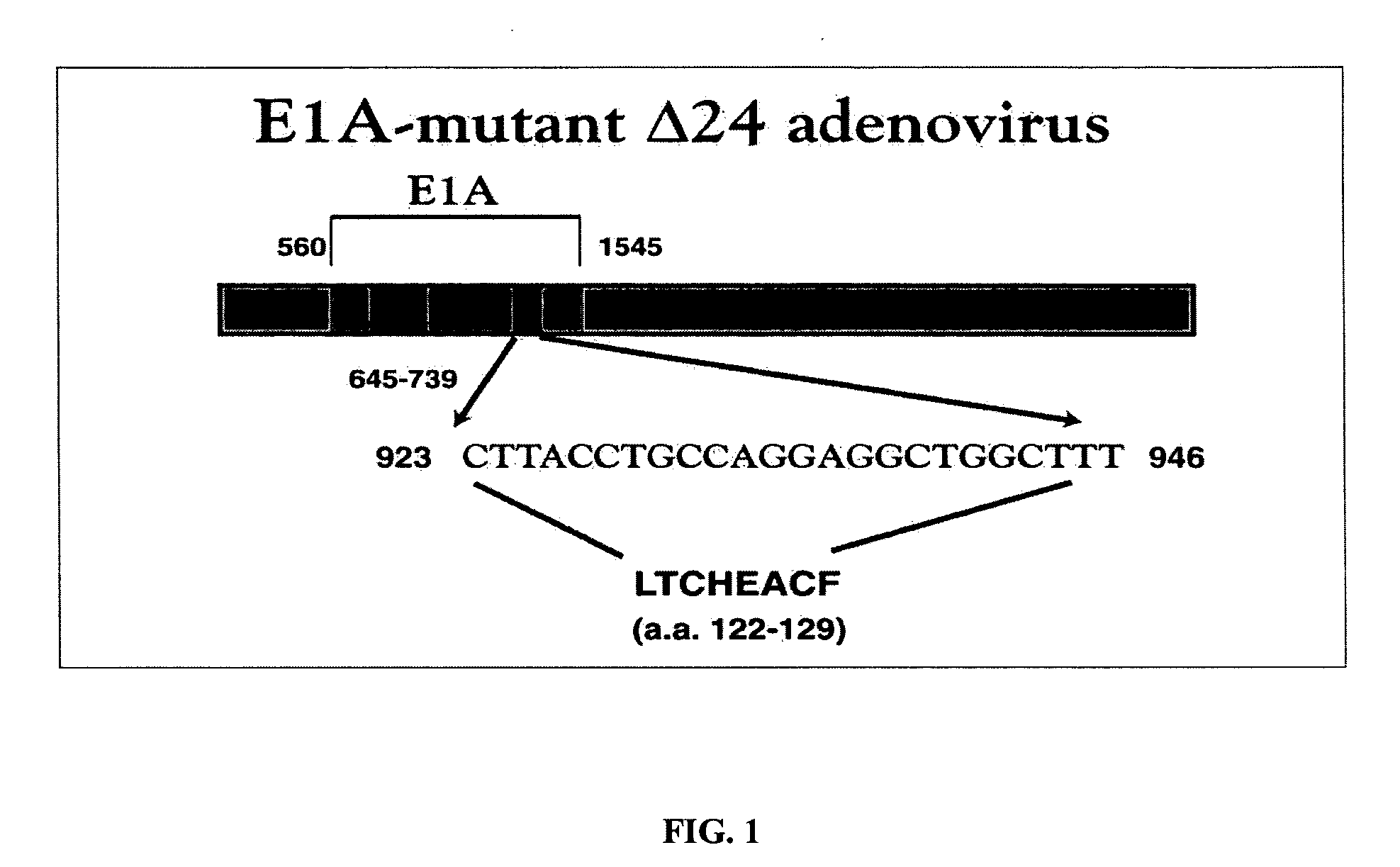
Oncolytic adenovirus armed with therapeutic genes
a technology of oncolytic adenovirus and therapeutic genes, which is applied in the field of oncolytic adenoviruses, can solve the problems that the therapeutic effect of the gene in vivo is limited, the tumors are not significant enough to be treated with existing adenovirus constructs, and the cell's regulatory machinery for controlling growth is upset, etc., to achieve the effect of greater efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
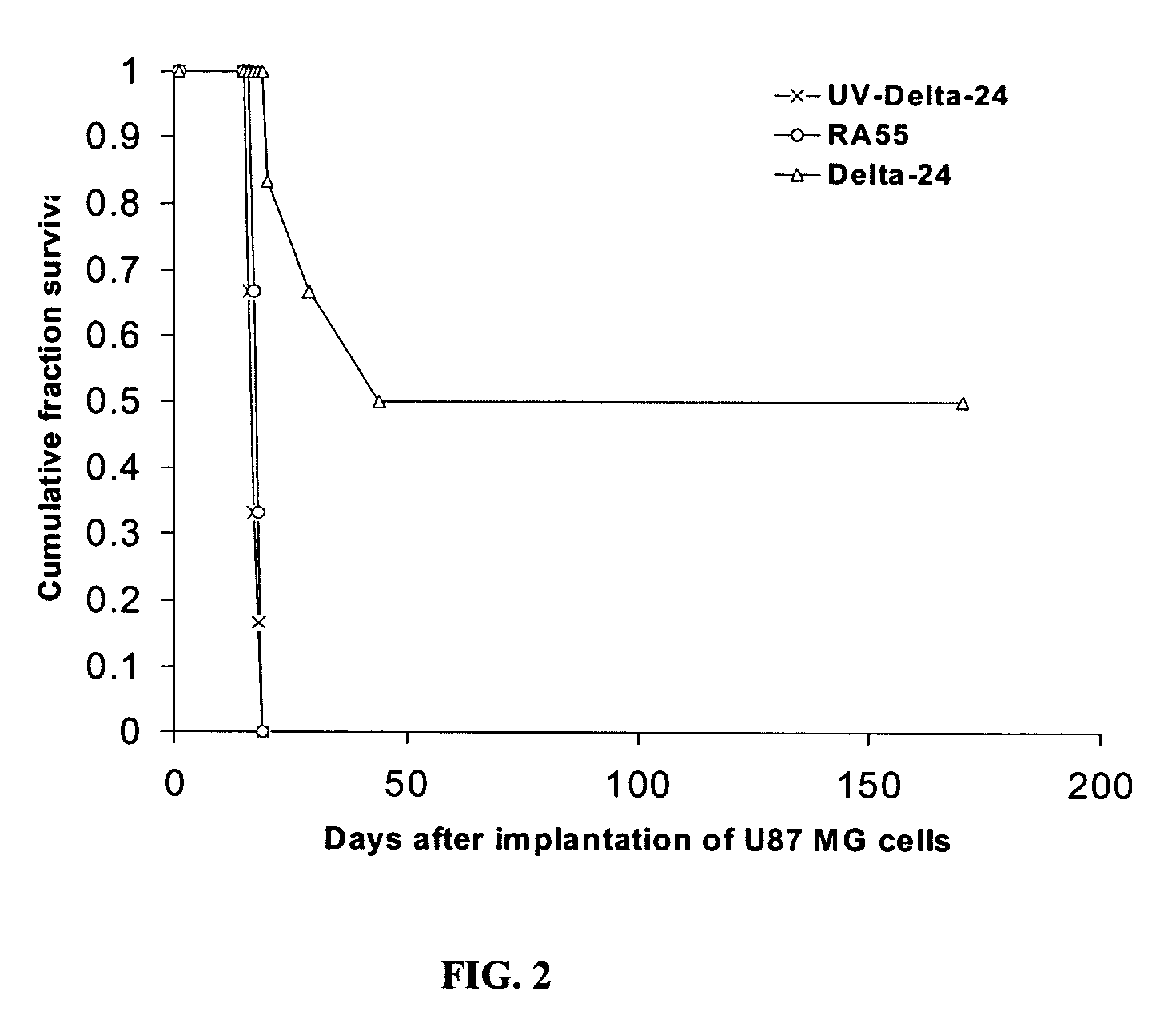
Delta 24 Studies
A. Material and Methods
[0259] Regional Glioma Models. U87MG and U251MG glioma cell human xenograft models utilize human tumor cell lines obtained from patients, which are placed into short or long-term tissue culture. The advantages of this system are that the tumor cells are of human origin and have readily identifiable characteristics such as expression levels of various growth factors and receptors. These studies, however, require immunocompromised animals to prevent tumor rejection, eliminating analysis of the role of the immune system in tumor biology and response to treatments. Most xenograft models are well established and form predictable and reliable intracranial tumors with fairly uniform animal deaths occurring at 20-22 days. However, these tumors lack the heterogeneity seen in the clinical setting. Furthermore, most xenograft models do not demonstrate extensive invasion of the surrounding brain parenchyma. The implanted tumors tend to grow spherically ...
example 2
Construction and Characterization of Delta 24-NIS
[0275] To determine whether the expression pattern of NIS is controlled by the status of the Rb pathway in Δ24-NIS-infected glioma cells the inventors studied the correlation between an E2F-promoter driven NIS expression with the replication ability of Δ24 in glioma cells and normal human astrocytes with different cell cycle profiles (from quiescence to active proliferation). The expression of NIS under the control of the tumor-specific promoter hTERT was also studied. Furthermore, the capability of Δ24-NIS infected cells to effectively take up various radionuclides was characterized.
A. Material and Methods
[0276] Materials and Methods used in these studies include the construction and characterization of a replication-competent Δ24 adenovirus encoding a therapeutic or diagnostic transgene, e.g., Δ24-hyCD or Δ24-NIS, see schematic representation shown in FIG. 13. Also described are the material and methods used for assessing transg...
example 3
Characterization of in vivo Effects of Radionuclide Accumulation for Imaging Tumors
[0296] These studies are conducted to determine the level of Δ24-NIS propagation in intracranial glioma tumor-bearing animals through the imaging of various radionuclides, to correlate imaging at multiple time points with pathologic material to determine regional aspects of viral spread throughout tumors both in intracranial gliomas and systemic lung cancer models with liposome:Δ24-NIS complexes, and to determine, by in vivo imaging, the efficiency and specificity of delivery of Δ24-NIS mediated mesenchymal stem cell deliverv to a systemic lung cancer model.
A. Characterizing the in vivo Effects of Radionuclide Accumulation for Imaging Tumors
[0297] It is critical to the future of oncolytic gene therapy trials to have the ability to monitor the infective spread of virus throughout a tumor. A sensitive and convenient method is needed to image this viral progress. This may have implications in the fut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com