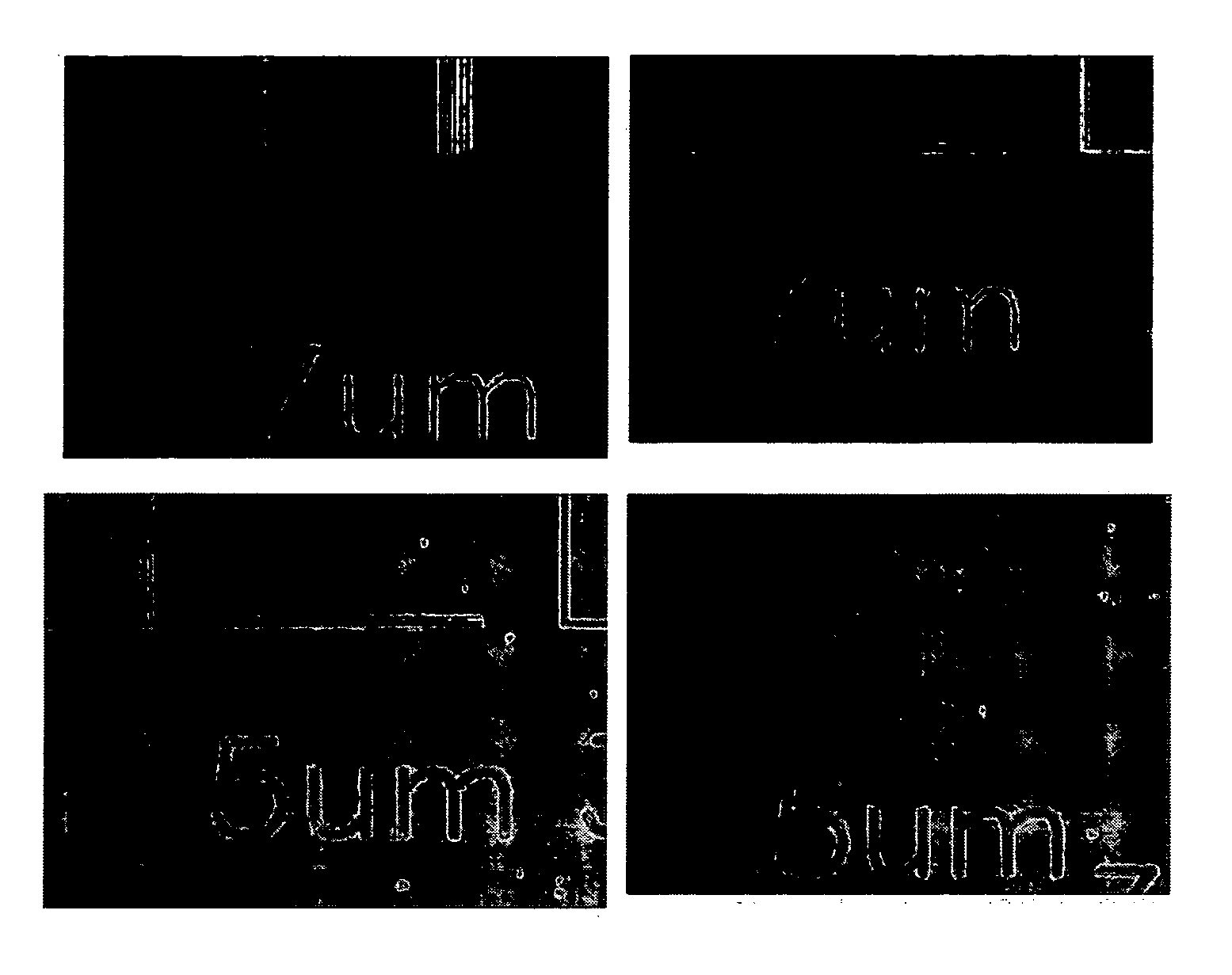
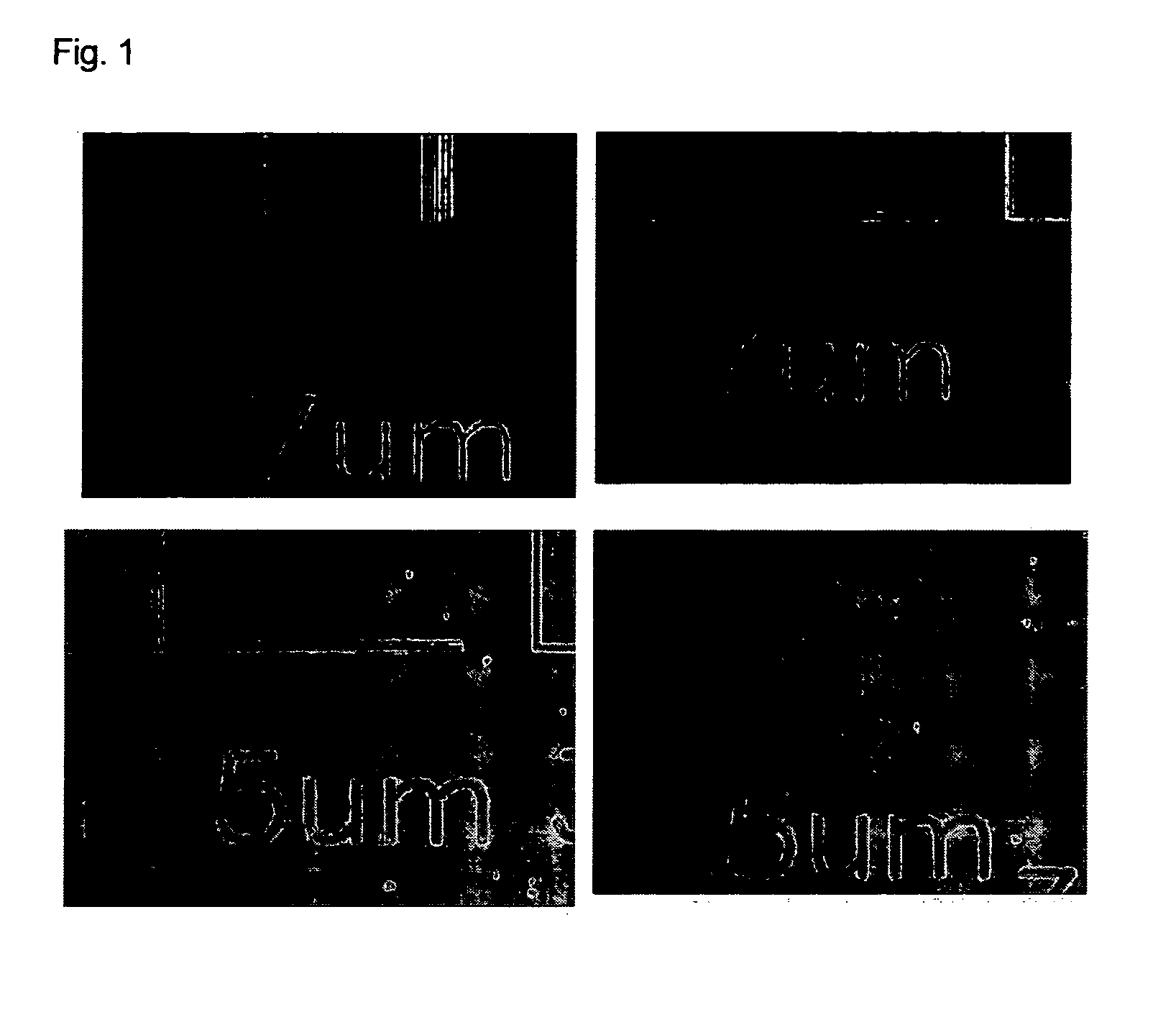
Norbornene polymer for photoresist and photoresist composition comprising the same
a technology of norbornene polymer and composition, which is applied in the direction of photosensitive materials, auxillary/base layers of photosensitive materials, instruments, etc., can solve the problems of affecting the composition, affecting the composition, and affecting the composition, etc., to achieve the effect of improving light transmittance, improving mechanical and thermal properties, and low dielectric constan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
PREPARATIVE EXAMPLE 2
Synthesis of Poly(tBN-co-GlyN-co-MA)
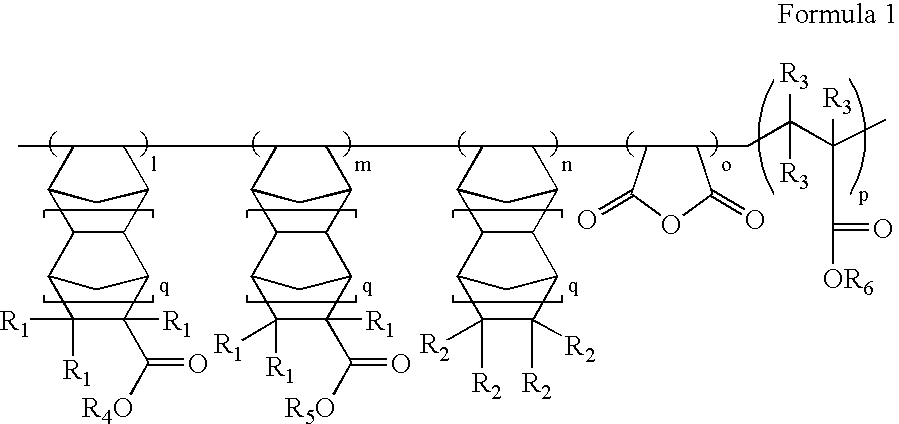
[0049] 15.0 g (77.2 mmol) of t-butyl norbomene carboxylate (tBN), 6.42 g (33.1 mmol) of glycidyl norbomene carboxylate (GlyN), 10.81 g (110.3 mmol) of purified maleic anhydride (MA), and 0.362 g (2.2 mmol) of 2,2′-azobisisobutyronitrile (AIBN) as a polymerization initiator were placed in a polymerization flask, and 33 g of purified tetrahydrofuran was added thereto. The resulting solution was polymerized according to the same procedure as in Preparative Example 1 to give 11.86 g (yield: 37%) of the target polymer (Mw =6,520) as a white solid. The target polymer has a formula according to Formula 4, below:
example 3
PREPARATIVE EXAMPLE 3
Synthesis of Poly(tBN-co-GlyN-co-NB-co-MA-co-tBMA)
[0050] 5.0 g (25.7 mmol) of t-butyl norbomene carboxylate (tBN), 6.66 g (34.3 mmol) of glycidyl norbomene carboxylate (GlyN), 3.22 g (34.2 mmol) of norbomene (NB), 8.41 g (85.8 mmol) of purified maleic anhydride (MA), 3.66 g (25.7 mmol) of t-butyl methacrylate, and 0.20 g (1.2 mmol) of 2,2′-azobisisobutyronitrile (AIBN) as a polymerization initiator were placed in a polymerization flask, and 27 g of purified tetrahydrofuran was added thereto. The resulting solution was polymerized according to the same procedure as in Preparative Example 1 to give 18.86 g (yield: 70%) of the target polymer (Mw=10,800) as a white solid. The target polymer has a formula according to Formula 5, below:
example 4
PREPARATIVE EXAMPLE 4
Synthesis of Poly(NCA-co-GlyN-co-NB-co-DEMMA)
[0051] 2.23 g (16.14 mmol) of 5-norbomene-2-carboxylic acid (NCA), 6.26 g (32.2 mmol) of glycidyl norbomene carboxylate (GlyN), 6.26 g (66.5 mmol) of norbomene (NB), 18.69 g (80.5 mmol) of a maleate monomer (DEMMA) prepared by the reaction of chloromethylethyl ether and maleic acid, and 0.32 g (1.95 mmol) of 2,2′-azobisisobutyronitrile (AIBN) as a polymerization initiator were placed in a polymerization flask, and then 34 g of purified tetrahydrofuran was added thereto. The resulting solution was polymerized according to the same procedure as in Preparative Example 1 to give 14.05 g (yield: 42%) of the target polymer (Mw=6,200) as a white solid. The target polymer has a formula according to Formula 6, below:
PUM
| Property | Measurement | Unit |
|---|---|---|
| photosensitive | aaaaa | aaaaa |
| mechanical | aaaaa | aaaaa |
| thermal properties | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com