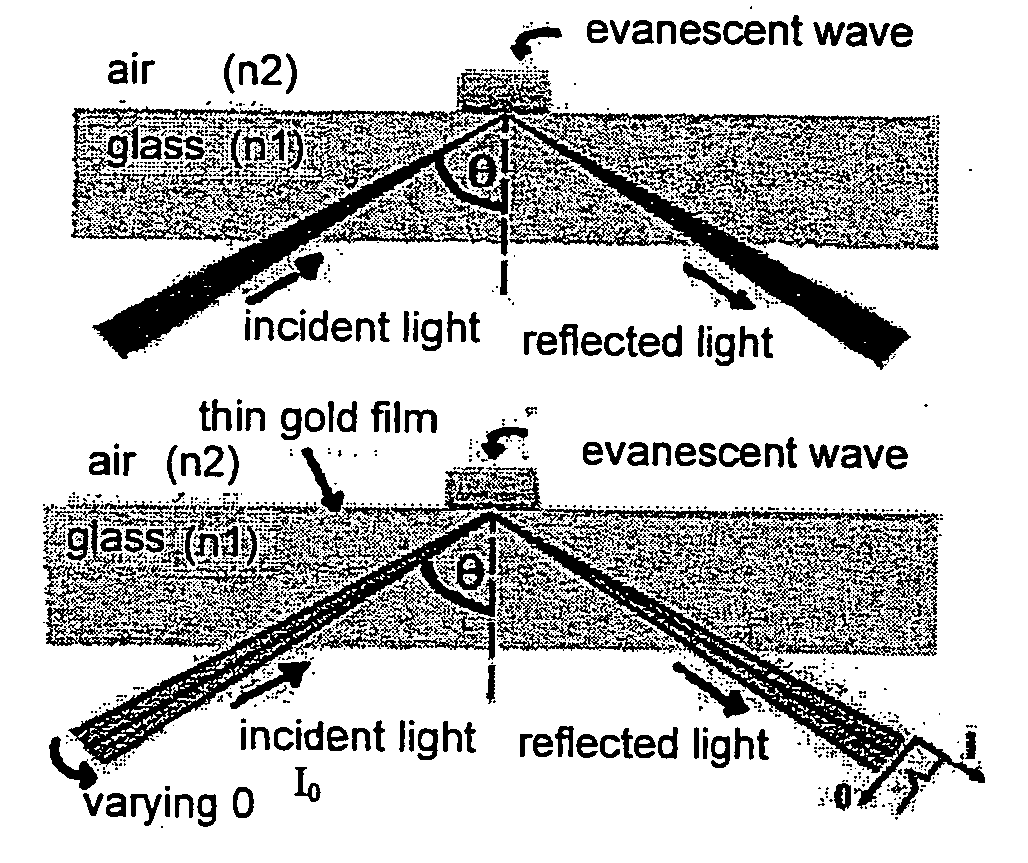
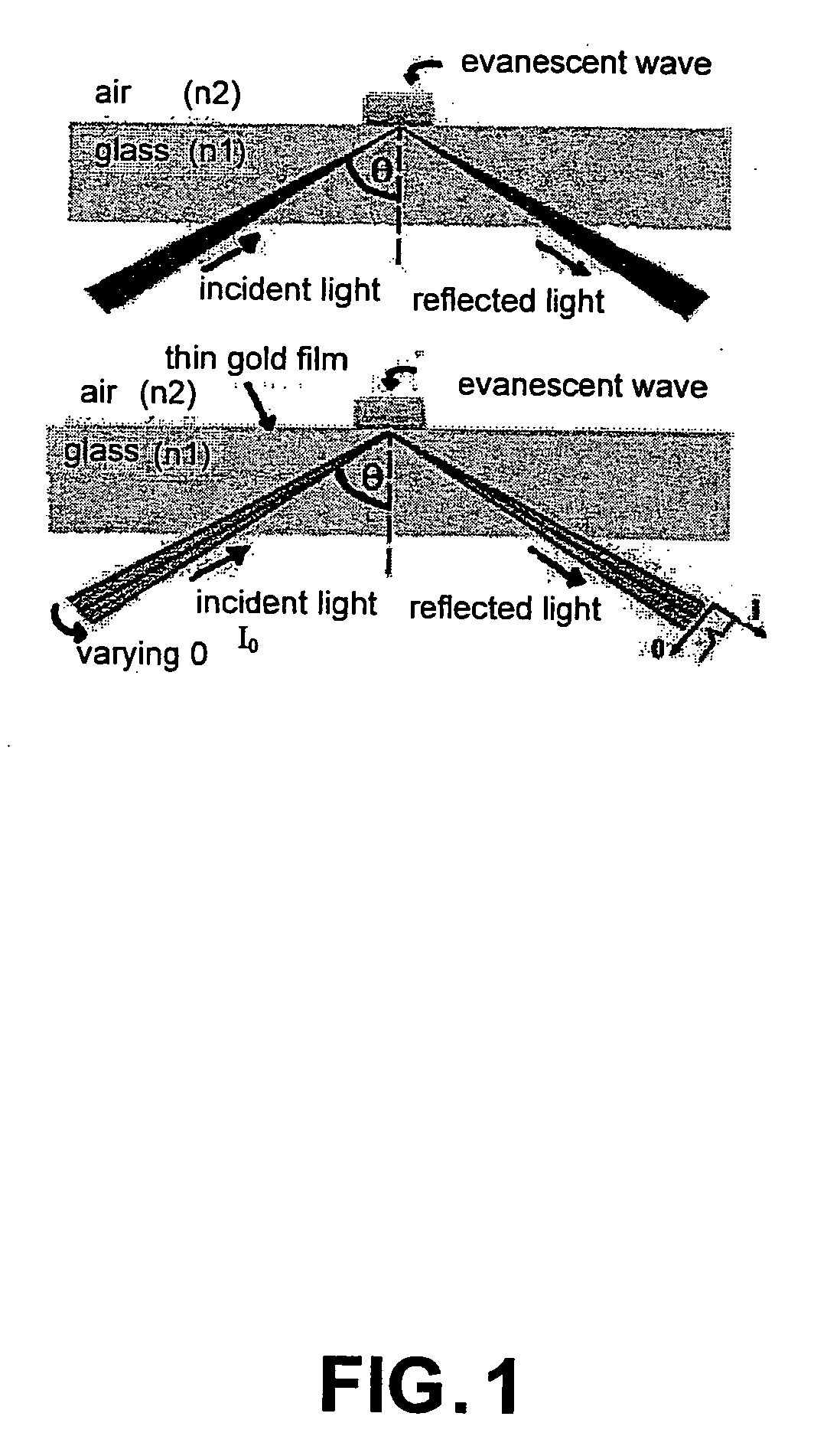
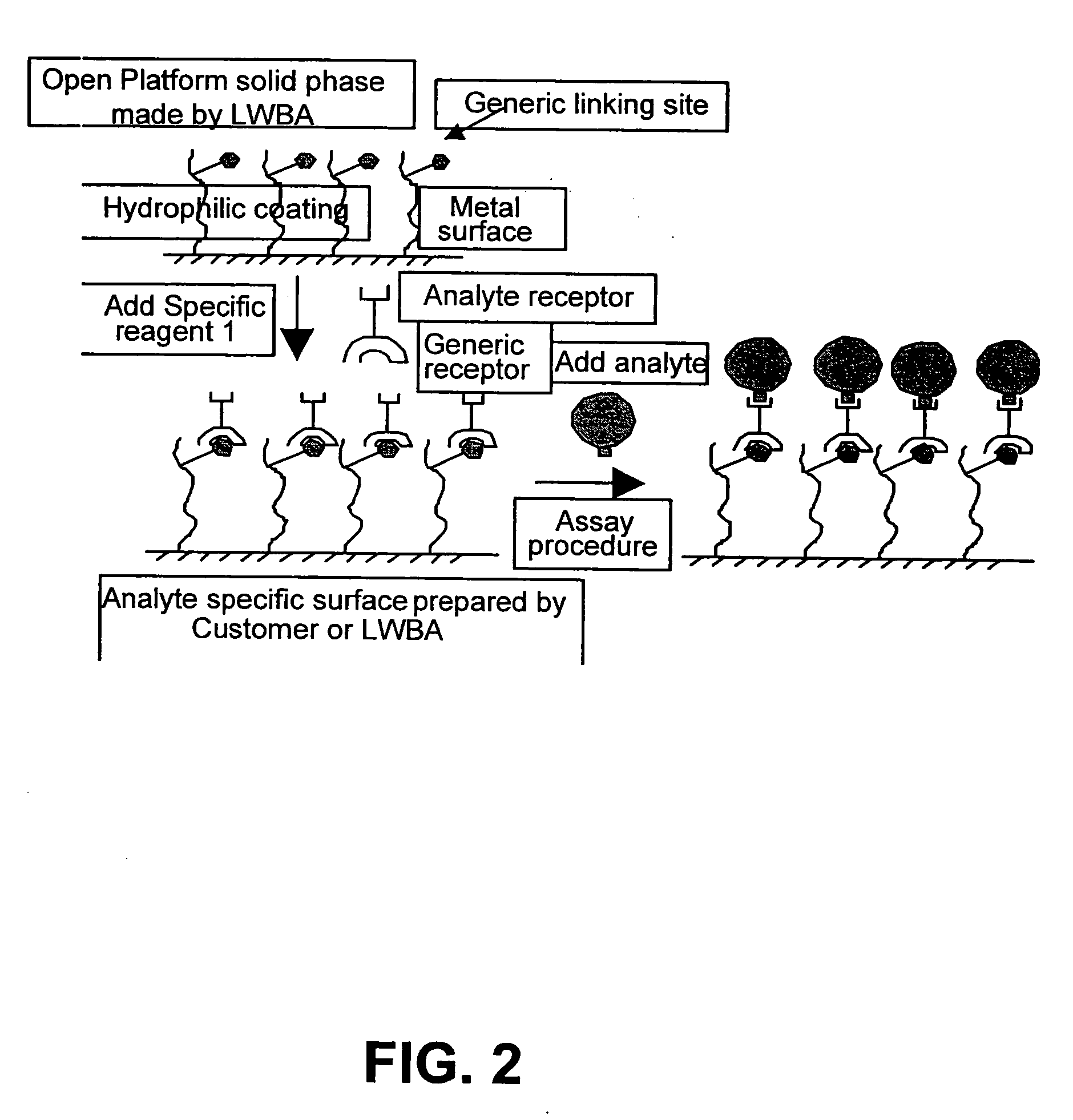
Bioanalysis systems including optical integrated circuit
a bioanalysis and integrated circuit technology, applied in the field of bioanalysis systems and methods, can solve the problems of inhibiting its wide spread use, difficult and inefficient current methods used in the identification and validation of “targets” and the optimization of drug structure in the pharmaceutical industry, and requiring vast sums of money and inordinate amounts of time, so as to achieve high throughput, and reduce the effect of drug discovery and developmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Receptor Coated Gold Surface
[0152] Gold surfaces are prepared and washed using (1) water, (2) ethanol. The gold surfaces are then exposed to at least 5 μL / mm2 of 10 mM 11-mercaptoundecanoic acid (MUA) dissolved in ethanol overnight at RT. The surfaces are then washed using (1) ethanol, (2) 50% ethanol / water, (3) 25% ethanol / water, (4) water then dried. The MUA treated gold surface is then exposed to at least 5 μL / mm2 of 100 mM N-ethyl-N1-(dimethylaminopropyl)carbodiimide (EDCI) and 40 mM N-Hydroxysuccinimide (NHS) in 50 mM MES-Na, at pH 5.0 at RT for 30 min to form an NHS-ester of the MUA. The NHS-ester surfaces are washed with PBS (Phosphate buffered saline) then exposed to streptavidin (10 μg / mL) in PBS for 30 min at RT. Finally the gold surface linked to streptavidin is washed with PBS.
example 2
Incorporation of a Receptor into a Microwell Array
[0153] The gold surfaces of the microwell array are coupled to streptavidin according to the procedure of Example 1.
example 3
Incorporation of a Receptor into a 2 Dimensional Array
[0154] Streptavidin is coupled to the gold surface of a 2 dimensional array constructed using the method of Example 1. A set of biotinylated receptors (prepared by literature methods) is bound to the streptavidin coated surface by “printing” small volumes of receptor solutions (0.5 μL of each at 10 μg / mL in PBS) at known locations on the surface spaced apart by more than 2 mm. After a brief incubation (2 min), unbound receptors are removed by washing the array with PBS.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com